MEG-based neurofeedback for hand rehabilitation
- PMID: 26392353
- PMCID: PMC4578759
- DOI: 10.1186/s12984-015-0076-7
MEG-based neurofeedback for hand rehabilitation
Abstract
Background: Providing neurofeedback (NF) of motor-related brain activity in a biologically-relevant and intuitive way could maximize the utility of a brain-computer interface (BCI) for promoting therapeutic plasticity. We present a BCI capable of providing intuitive and direct control of a video-based grasp.
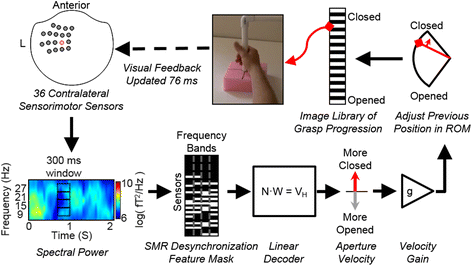
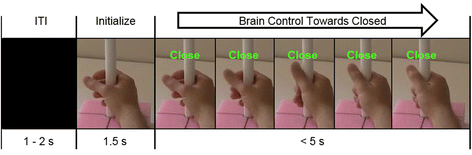
Methods: Utilizing magnetoencephalography's (MEG) high temporal and spatial resolution, we recorded sensorimotor rhythms (SMR) that were modulated by grasp or rest intentions. SMR modulation controlled the grasp aperture of a stop motion video of a human hand. The displayed hand grasp position was driven incrementally towards a closed or opened state and subjects were required to hold the targeted position for a time that was adjusted to change the task difficulty.
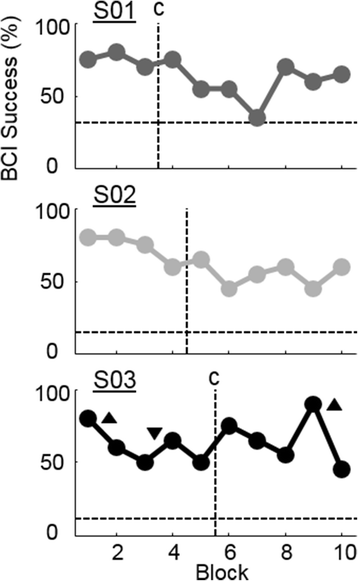
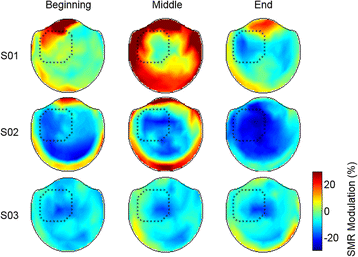
Results: We demonstrated that three individuals with complete hand paralysis due to spinal cord injury (SCI) were able to maintain brain-control of closing and opening a virtual hand with an average of 63 % success which was significantly above the average chance rate of 19 %. This level of performance was achieved without pre-training and less than 4 min of calibration. In addition, successful grasp targets were reached in 1.96 ± 0.15 s. Subjects performed 200 brain-controlled trials in approximately 30 min excluding breaks. Two of the three participants showed a significant improvement in SMR indicating that they had learned to change their brain activity within a single session of NF.
Conclusions: This study demonstrated the utility of a MEG-based BCI system to provide realistic, efficient, and focused NF to individuals with paralysis with the goal of using NF to induce neuroplasticity.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

