Granzyme B-Mediated Activation-Induced Death of CD4+ T Cells Inhibits Murine Acute Graft-versus-Host Disease
- PMID: 26392464
- PMCID: PMC4610854
- DOI: 10.4049/jimmunol.1500668
Granzyme B-Mediated Activation-Induced Death of CD4+ T Cells Inhibits Murine Acute Graft-versus-Host Disease
Abstract
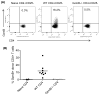
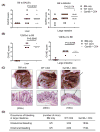
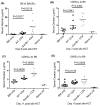
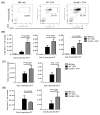
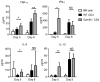
Granzyme B (GzmB) has previously been shown to be critical for CD8(+) T cell-mediated graft-versus-host disease (GVHD) but dispensable for GVHD mediated by CD4(+) T cells. However, previous studies used high doses of CD4(+) T cells in MHC-mismatched models that caused rapid and lethal GVHD. Because of the hyperacute lethality, it is possible that the role of GzmB was concealed by the system. Therefore, in this study, we have titrated down the T cell dose to precisely determine the contribution of GzmB in GVHD mediated by CD4(+)CD25(-) T cells. Surprisingly, we have found that GzmB(-/-)CD4(+)CD25(-) T cells cause more severe GVHD compared with wild-type CD4(+)CD25(-) T cells in both MHC-matched and mismatched models. Mechanistic analyses reveal that although GzmB does not affect donor T cell engraftment, proliferation or tissue-specific migration, GzmB(-/-) CD4(+)CD25(-) T cells exhibit significantly enhanced expansion because of GzmB-mediated activation-induced cell death of wild-type CD4(+)CD25(-) T cells. As a result of enhanced expansion, GzmB(-/-) T cells produced higher amounts of proinflammatory cytokines (e.g., TNF-α and IFN-γ) that may contribute to the exacerbated GVHD. These results reveal that GzmB diminishes the ability of CD4(+) T cells to cause acute GVHD, which contradicts its established role in CD8(+) T cells. The differential roles suggest that targeting GzmB in selected T cell subsets may provide a strategy to control GVHD.
Copyright © 2015 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no potential conflict of interest to disclose.
Figures


References
-
- Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. - PubMed
-
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. - PubMed
-
- Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–170. - PubMed
-
- Vogelsang GB, Lee L, Bensen-Kennedy DM. Pathogenesis and treatment of graft-versus-host disease after bone marrow transplant. Annu Rev Med. 2003;54:29–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

