The Nucleoside Analog BMS-986001 Shows Greater In Vitro Activity against HIV-2 than against HIV-1
- PMID: 26392486
- PMCID: PMC4649195
- DOI: 10.1128/AAC.01326-15
The Nucleoside Analog BMS-986001 Shows Greater In Vitro Activity against HIV-2 than against HIV-1
Abstract
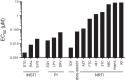
Treatment options for individuals infected with human immunodeficiency virus type 2 (HIV-2) are restricted by the intrinsic resistance of the virus to nonnucleoside reverse transcriptase inhibitors (NNRTIs) and the reduced susceptibility of HIV-2 to several protease inhibitors (PIs) used in antiretroviral therapy (ART). In an effort to identify new antiretrovirals for HIV-2 treatment, we evaluated the in vitro activity of the investigational nucleoside analog BMS-986001 (2',3'-didehydro-3'-deoxy-4'-ethynylthymidine; also known as censavudine, festinavir, OBP-601, 4'-ethynyl stavudine, or 4'-ethynyl-d4T). In single-cycle assays, BMS-986001 inhibited HIV-2 isolates from treatment-naive individuals, with 50% effective concentrations (EC50s) ranging from 30 to 81 nM. In contrast, EC50s for group M and O isolates of HIV-1 ranged from 450 to 890 nM. Across all isolates tested, the average EC50 for HIV-2 was 9.5-fold lower than that for HIV-1 (64 ± 18 nM versus 610 ± 200 nM, respectively; mean ± standard deviation). BMS-986001 also exhibited full activity against HIV-2 variants whose genomes encoded the single amino acid changes K65R and Q151M in reverse transcriptase, whereas the M184V mutant was 15-fold more resistant to the drug than the parental HIV-2ROD9 strain. Taken together, our findings show that BMS-986001 is an effective inhibitor of HIV-2 replication. To our knowledge, BMS-986001 is the first nucleoside analog that, when tested against a diverse collection of HIV-1 and HIV-2 isolates, exhibits more potent activity against HIV-2 than against HIV-1 in culture.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- United States Department of Health and Human Services. 4 May 2015, posting date HIV/AIDS fact sheets: FDA-approved HIV medicines. United States Department of Health and Human Services, Washington, DC: http://aidsinfo.nih.gov/education-materials/fact-sheets/19/58/fda-approv....
-
- May MT, Gompels M, Delpech V, Porter K, Orkin C, Kegg S, Hay P, Johnson M, Palfreeman A, Gilson R, Chadwick D, Martin F, Hill T, Walsh J, Post F, Fisher M, Ainsworth J, Jose S, Leen C, Nelson M, Anderson J, Sabin C. 2014. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS 28:1193–1202. doi:10.1097/QAD.0000000000000243. - DOI - PMC - PubMed
-
- Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, Burchell AN, Cohen M, Gebo KA, Gill MJ, Justice A, Kirk G, Klein MB, Korthuis PT, Martin J, Napravnik S, Rourke SB, Sterling TR, Silverberg MJ, Deeks S, Jacobson LP, Bosch RJ, Kitahata MM, Goedert JJ, Moore R, Gange SJ. 2013. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 8:e81355. doi:10.1371/journal.pone.0081355. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

