Optimization of Single- and Dual-Color Immunofluorescence Protocols for Formalin-Fixed, Paraffin-Embedded Archival Tissues
- PMID: 26392518
- PMCID: PMC4812679
- DOI: 10.1369/0022155415610792
Optimization of Single- and Dual-Color Immunofluorescence Protocols for Formalin-Fixed, Paraffin-Embedded Archival Tissues
Abstract
Performance of immunofluorescence staining on archival formalin-fixed paraffin-embedded human tissues is generally not considered to be feasible, primarily due to problems with tissue quality and autofluorescence. We report the development and application of procedures that allowed for the study of a unique archive of thymus tissues derived from autopsies of individuals exposed to atomic bomb radiation in Hiroshima, Japan in 1945. Multiple independent treatments were used to minimize autofluorescence and maximize fluorescent antibody signals. Treatments with NH3/EtOH and Sudan Black B were particularly useful in decreasing autofluorescent moieties present in the tissue. Deconvolution microscopy was used to further enhance the signal-to-noise ratios. Together, these techniques provide high-quality single- and dual-color fluorescent images with low background and high contrast from paraffin blocks of thymus tissue that were prepared up to 60 years ago. The resulting high-quality images allow the application of a variety of image analyses to thymus tissues that previously were not accessible. Whereas the procedures presented remain to be tested for other tissue types and archival conditions, the approach described may facilitate greater utilization of older paraffin block archives for modern immunofluorescence studies.
Keywords: archival tissue; image analysis; immunofluorescence; thymus; tissue processing.
© 2016 The Histochemical Society.
Conflict of interest statement
Figures
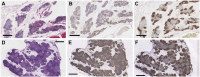
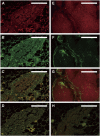
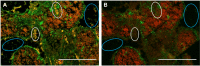
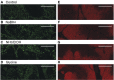
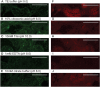


References
-
- Baschong W, Suetterlin R, Laeng RH. (2001). Control of autofluorescence of archival formaldehyde-fixed, paraffin-embedded tissue in confocal laser scanning microscopy (CLSM). J Histochem Cytochem 49:1565-1572. - PubMed
-
- Beisker W, Dolbeare F, Gray JW. (1987). An improved immunocytochemicalprocedure for high-sensitivity detection of incorporated bromodeoxyuridine. Cytometry 8:235-239. - PubMed
-
- Kiernan JA. (2008). Histological and Histochemical Methods: Theory and Practice. 4th edition, Bloxham: Scion.
-
- Oliveira VC, Carrara RC, Simoes DL, Saggioro FP, Carlotti CG, Covas DT, Neder L. (2010). Sudan Black B treatment reduced autofluorescence and improves resolution of in situ hybridization specific fluorescent signals of brain sections. Histol Histopathol 25:1017-1024. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

