Thermodynamics of protein destabilization in live cells
- PMID: 26392565
- PMCID: PMC4603463
- DOI: 10.1073/pnas.1511308112
Thermodynamics of protein destabilization in live cells
Abstract
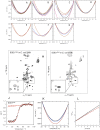
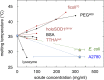
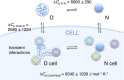
Although protein folding and stability have been well explored under simplified conditions in vitro, it is yet unclear how these basic self-organization events are modulated by the crowded interior of live cells. To find out, we use here in-cell NMR to follow at atomic resolution the thermal unfolding of a β-barrel protein inside mammalian and bacterial cells. Challenging the view from in vitro crowding effects, we find that the cells destabilize the protein at 37 °C but with a conspicuous twist: While the melting temperature goes down the cold unfolding moves into the physiological regime, coupled to an augmented heat-capacity change. The effect seems induced by transient, sequence-specific, interactions with the cellular components, acting preferentially on the unfolded ensemble. This points to a model where the in vivo influence on protein behavior is case specific, determined by the individual protein's interplay with the functionally optimized "interaction landscape" of the cellular interior.
Keywords: NMR; crowding; in vivo; protein stability; thermodynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

