Formation of the transition zone by Mks5/Rpgrip1L establishes a ciliary zone of exclusion (CIZE) that compartmentalises ciliary signalling proteins and controls PIP2 ciliary abundance
- PMID: 26392567
- PMCID: PMC4609185
- DOI: 10.15252/embj.201488044
Formation of the transition zone by Mks5/Rpgrip1L establishes a ciliary zone of exclusion (CIZE) that compartmentalises ciliary signalling proteins and controls PIP2 ciliary abundance
Abstract
Cilia are thought to harbour a membrane diffusion barrier within their transition zone (TZ) that compartmentalises signalling proteins. How this "ciliary gate" assembles and functions remains largely unknown. Contrary to current models, we present evidence that Caenorhabditis elegans MKS-5 (orthologue of mammalian Mks5/Rpgrip1L/Nphp8 and Rpgrip1) may not be a simple structural scaffold for anchoring > 10 different proteins at the TZ, but instead, functions as an assembly factor. This activity is needed to form TZ ultrastructure, which comprises Y-shaped axoneme-to-membrane connectors. Coiled-coil and C2 domains within MKS-5 enable TZ localisation and functional interactions with two TZ modules, consisting of Meckel syndrome (MKS) and nephronophthisis (NPHP) proteins. Discrete roles for these modules at basal body-associated transition fibres and TZ explain their redundant functions in making essential membrane connections and thus sealing the ciliary compartment. Furthermore, MKS-5 establishes a ciliary zone of exclusion (CIZE) at the TZ that confines signalling proteins, including GPCRs and NPHP-2/inversin, to distal ciliary subdomains. The TZ/CIZE, potentially acting as a lipid gate, limits the abundance of the phosphoinositide PIP2 within cilia and is required for cell signalling. Together, our findings suggest a new model for Mks5/Rpgrip1L in TZ assembly and function that is essential for establishing the ciliary signalling compartment.
Keywords: MKS5; PIP2; cilia; ciliary gate; transition zone.
© 2015 The Authors.
Figures

Schematic depicting phasmid (tail) cilia at dendritic tips (DTs), including basal body-associated transition fibres (TFs) and transition zone (TZ).
TMEM-17::EGFP and TMEM-138::EGFP localise to the TZ in some, but not all, TZ mutants indicated; lack of localisation is denoted by a dotted ellipse. The co-marker tdTomato-tagged XBX-1 marks TFs and the ciliary axoneme.
MKS and NPHP protein localisation in mks-2 and tmem-17 mutants. In mks-2 animals, MKS-1, MKSR-1, MKSR-2, MKS-3, MKS-6, and TMEM-17 are delocalised (dotted ellipse) but NPHP-1, NPHP-4, and MKS-5 remain at the TZ. All tested TZ proteins remain at the TZ of the tmem-17 mutant.
NPHP-4 and NPHP-1 localise to both the TZ and TFs in wild-type animals, but are only found at TFs in the mks-5 mutant. The CHE-13 reporter shown marks TFs and the entire axoneme. Note the specific overlap (see merge) between NPHP-1 and NPHP-4 with the TF in the mks-5 mutant.
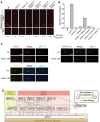
Disruption of tmem-17 alone does not cause overt ciliary defects as measured by uptake of fluorescent dye through environmentally exposed cilia. tmem-17;nphp-4 double mutants are defective in dye uptake while tmem-17;mks-2,tmem-17;mksr-1 and tmem-17;mks-3 take up the dye similar to tmem-17 alone. Scale bar: 20 μm.
tmem-17 mutants avoid high-osmolarity solutions, as do tmem-17;mks-2,tmem-17;mksr-1, and tmem-17;mks-3 double mutants. tmem-17;nphp-4 double mutants are partially defective in sensing osmolarity, though not as severely as osm-5 (IFT mutant) animals, which are completely oblivious of the hyperosmotic barrier (*P < 0.0001, chi-squared test).
TZ markers expressed in wild-type and the tmem-138 mutants. MKS-5, TMEM-231 (MKS module), and NPHP-4 (NPHP module) do not require TMEM-138 for TZ localisation. Scale bars: 4 μm.
Model showing all of the negative interaction amongst MKS/NPHP proteins, where a particular MKS/NPHP protein (at the arrowhead) is localised properly in a specific TZ mutant; a white circle on the arrowhead indicates new data obtained in this study.

mks-2;nphp-4 mutants display a failure to uptake the lipophilic dye DiI which can be rescued by the wild-type MKS-2::GFP reporter. Shown are transgenic animals (arrowheads) that express the reporter and uptake of the dye, while their nontransgenic siblings cannot uptake the dye. Scale bar: 100 μm.
tmem-17;nphp-4 mutants display a failure to uptake the lipophilic dye DiI which can be rescued by the wild-type TMEM-17::GFP translational reporter. Shown are transgenic animals (arrowheads) that express the reporter and uptake of the dye, while their nontransgenic siblings cannot uptake the dye. Scale bar: 100 μm.
The mks-5(gk153561);nphp-4 mutants display a Dyf phenotype, which can be rescued with expression of the wild-type MKS-5::tdTomato translational reporter. In the third set of head and tail panels, transgenic worms are shown at the same exposure time, but the signal is not detectable. In the right-hand set of panels, a tenfold longer exposure shows detectable signal for the MKS-5::tdTomato reporter, indicating that the signal for the transgenic worms is coming from the dye. Scale bar: 20 μm.
Scatter plots of measured IFT velocities for wild-type and mks-5 mutants using the CHE-13::YFP translational reporter. In the transition zone (TZ; proximal ˜0.8 μm of the cilium), the mean IFT velocity is 0.39 μm/s in wild-type animals, but increases to 0.49 μm/s in the mks-5 mutant, which lacks a detectable TZ. In the middle segment, mean IFT velocity is 0.69 μm/s, while in the mks-5 mutant, it is significantly slower at 0.63 μm/s. *P < 0.001, t-test.
IFT flux (in particles per minute) is shown for wild-type and mks-5 mutants. No difference is observed between the flux of IFT particles in wild-type and mks-5 animals (P > 0.05, t-test).

Transmission electron microscopy (TEM) serial cross-section images of wild-type and mks-5(tm3100) amphid channel cilia. Boxed numbers denote proximal positioning of section relative to left-most (anterior) section of the animal. Wild-type amphid channels contain 10 axonemes, each possessing the following: a distal segment (DS) with singlet microtubules (MTs); a middle segment (MS) with doublet MTs and inner singlet MTs; a transition zone (TZ) with Y-link connectors, and doublet MT-associated apical ring; and a basal body/transition fibre (TF) region. mks-5 worms possess grossly normal DSs (although one axoneme is frequently missing), and MSs that frequently display MT singlets and doublets reduced in number and occasionally displaced (see high-magnification images). TZs are not observed in mks-5 worms, replaced instead by abnormal middle segments (labelled “MS?”). Scale bars: 200 nm (low-magnification images); 100 nm (high-magnification images).
The TZ region of mks-5 mutants lacks all distinguishing features, including the apical ring (arrow), Y-links (closed arrowhead), and tight connection to the ciliary membrane. Doublet MTs of mks-5 mutant TZs are often displaced. Open arrowhead: inner singlet MT. Scale bars: 100 nm.
Schematics summarising ultrastructures of wild-type and mks-5 mutants. PCMC, periciliary membrane compartment.

A tmem-17 mutant cilium ultrastructure is similar to wild-type worms, possessing all characteristic features, including singlet microtubules (MTs) in the distal segments (DSs), an ordered ring of doublet MTs surrounding internal singlet MTs in the middle segments (MSs), and transition zones (TZ) possessing Y-links and an apical ring tethering inner singlet MTs to outer doublet MTs. Scale bars: 200 nm (low-mag. images); 100 nm (high-mag. images).
B, C In tmem-17;nphp-4 double mutants, 3–4 middle and distal segments are missing and remaining axonemes sometimes possess missing doublet MTs. Most TZs lack some Y-link structure (closed arrowhead), and frequently other defects are observed, such as ectopic singlet MTs (closed arrow), reduced MT numbers (+7 image), and detachment from the ciliary/dendritic membrane (+7 image) indicative of an axoneme misplaced within the dendrite (see schematic). The apical membrane (open arrow) tethering inner singlet MTs (open arrowhead) to outer doublet MTs remains largely intact. Together, these ultrastructural defects are characteristic of mutants that carry a mutation in a member of both the NPHP module (nphp-4) and the MKS module (tmem-17). Scale bars: 200 nm (low-mag. images in B); 100 nm (high-mag. images in B and in C).
D Schematics summarising the ultrastructure phenotypes.

A mks-2 mutant cilium ultrastructure is mostly similar to wild-type worms, possessing all characteristic features, including singlet microtubules (MTs) in the distal segments (DSs), an ordered ring of doublet MTs surrounding internal singlet MTs in the middle segments (MSs), and transition zones (TZ) possessing Y-links and an apical ring tethering inner singlet MTs to outer doublet MTs. However, occasional axonemes possess abnormal numbers of singlet or doublet MTs (bottom-left image). Scale bar: 200 nm (low-mag. images); 100 nm (high-mag. images).
B, C In mks-2;nphp-4 double mutants, many middle and distal segments are missing. TZs completely lack Y-links and are detached from the ciliary/dendritic membrane indicative of axonemes anchored within the dendrite (i.e. misplaced; see schematic). The apical membrane that tethers inner singlet MTs to outer doublet MTs is difficult to discern, and doublet MTs are frequently disorganised (+10 image; left). Misplaced TZs are often surrounded by abnormal membrane (vesicle-like) accumulations (+5, +9, +10 images). These ultrastructural defects are characteristic of mutants that carry a mutation in both a member of the NPHP module (nphp-4) and the MKS module (mks-2). Scale bars: 200 nm (low-mag. images in B); 100 nm (high-mag. images in B and in C).
D Schematics summarising the ultrastructure phenotypes.


In wild-type worms, tdTomato-tagged MKS-5 localises to the transition zone (TZ) at a single obloid punctum. In the MKS module mutants mksr-1,mksr-2,mks-2,tmem-17,jbts-14,mks-3, and mks-6, as well as the double mutant mks-2;mksr-1, MKS-5 localises to two puncta at the TZ. MKS-5 localisation in the NPHP module mutants nphp-1 and nphp-4 and the double mutant nphp-1;nphp-4 displays another small point of localisation, distal to the TZ along the axoneme (arrowheads). Scale bar: 4 μm.
Mean length of the MKS-5 signal in the wild-type (wt), as well as mks-3 and mks-2 mutant strains. mks-2 mutants have disrupted MKS module protein localisation and are therefore more severe than mks-3, which do not affect localisation of any TZ proteins. The TZ is significantly longer in mks-3 mutants compared to wt, while in mks-2 it is significantly longer compared to either mks-3 or N2. *P < 0.001, t-test, error bars are 95% confidence interval.
Fraction of transition zones displaying two peaks of MKS-5 fluorescence. In wt, none of the TZ confocal images display two peaks. In mks-3 and mks-2 mutants, the proportion with two distinct fluorescence signals was significantly increased compared to wt. mks-2 mutants also showed significantly more TZs with two peaks compared to mks-3. *P < 0.001, chi-square test.
In double mutants with mutations in genes from each of the NPHP and MKS modules, mks-2;nphp-1 and mks-2;nphp-4, most cilia show MKS-5 localised not only to the TZ but often dispersing distally along some cilia. Additional phenotypes observed in these latter double mutants (short dendrite, long cilium, mis-oriented cilium) are shown. cb, cell body. Scale bar: 4 μm.

Schematic of predicted MKS-5 functional domains depicts 8 tandem coiled-coils, two C2 domains (C2-N and C2-C), and an Rpgr interaction domain (RID). Also shown are the missense alleles screened for synthetic dye-filling phenotypes (Dyf).
Schematics depict the localisation of the MKS-5 FL and truncation proteins. Top three panels: full-length (FL) MKS-5::tdTomato localises to the TZ, adjacent to the TF region marked by the IFT markers (DYF-11::EGFP or CHE-13::CFP). Loss of the first half of the coiled-coil region results in substantially reduced MKS-5 localisation to the TZ and accumulation at the dendritic tip. Removal of the entire coiled-coil region results in complete loss of TZ localisation. Bottom four panels: truncations of the C-terminal half, retaining the TZ-localising coiled-coils, were co-expressed in the mks-5 null with MKS-2 or NPHP-1. The MKS-5 full-length and truncation constructs lacking the RID both rescue NPHP-1 and MKS-2 TZ localisation. Removing the C2-C or both C2 domains rescues MKS-2 localisation but results in loss of NPHP-1 at the TZ (with NPHP-1 still retained at TFs). Schematics depict the localisation of the MKS-5 FL and truncation proteins. Scale bar: 4 μm.
Fluorescence images of dye-filling experiments show that one of the eight tested mks-5 alleles (gk153561), resulting in a D772N alteration, results in a synthetic Dyf in combination with nphp-4. None of the alleles, including gk153561, were Dyf when genetically crossed with mksr-1. A model mapping the position of D772 relative to the synaptotagmin-7 C2 domain crystal structure suggests that the aspartic acid residue lies within the β-sandwich interface, potentially altering the stability of the domain and disrupting calcium binding (shown as spheres) or lipid binding (not in the structure). Amino acid sequence alignment of the Caenorhabditis elegansMKS-5 C2-C domain with both H. sapiens (Hs) Rpgrip1 and Rpgrip1L shows conservation at D772. Scale bar: 20 μm.
No change in the localisation of NPHP-1::CFP (TF and TZ), NPHP-2::EGFP (inversin compartment), or MKS-2::EGFP (TZ) is detected in the mks-5(gk153561) mutants. Scale bar: 4 μm.
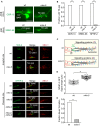
EGFP-tagged SRBC-66 and ODR-10 both localise to the entire axonemes of ASK and AWB neuron cilia, respectively, and are excluded from the TZ in wild type (wt); these markers occupy the entire cilium, including the TZ, in mks-5 mutants. TAX-4 and NPHP-2, orthologues of the cyclic nucleotide-gated channel CNGA1 and NPHP2/inversin respectively, which are found in the inversin (Inv) compartment in wt, also localise to the TZ in mks-5 animals. NPHP-2::EGFP was co-localised with XBX-1::tdTomato and are shown in the phasmid cilia while TAX-4::EGFP is shown in amphid cilia. den, dendrite; TZ, transition zone; BB, basal body; arrowheads indicate gap is present; * indicates no gap. Scale bars: 4 μm.
Quantification of cilia observed to have a clear gap in signal (i.e. CIZE) at the TZ in wt animals compared to mks-5 mutants for the ODR-10, SRBC-66, and NPHP-2 protein markers. *P < 0.001, chi-squared test.
Schematic depicting the CIZE, which excludes signalling proteins from the TZ in wt animals but not mks-5 animals, which lack TZ ultrastructure.
NPHP-2 signal length is longer in mks-5 animals compared to wt, consistent with the presence of an axonemal region that lacks TZ ultrastructure. *P < 0.001, t-test, error bars are standard deviation.
The mks-5 mutant displays a dauer formation phenotype compared to wild-type when presented with synthetic ASCR#2 dauer pheromone. ASCR#2 is detected by the SRBC-66 G-protein-coupled receptor, and downstream signalling includes the cGMP-dependent channel, TAX-4. *P < 0.001, chi-square test.
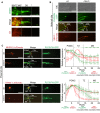
Co-localisation of the red fluorescent lipophilic dye DiI and OSM-5, a component of the IFT-B complex. GFP-tagged OSM-5 marks the basal body (BB), transition zone (TZ), and entire length of the amphid cilia axoneme. DiI co-localises with only the proximal or middle segment (MS) portion of the OSM-5 localisation, showing an exclusion of the dye from the distal segment (DS). This indicates that there is a barrier to DiI accumulation in the distal segment. Scale bar: 4 μm.
Co-localisation of GFP-tagged PLCδ-PH with tdTomato-tagged XBX-1 in the male tail rays shows enrichment, at the PCMC in wild-type animals (top panels, differential interference contrast (DIC) microscopy of male tail rays 8 and 9). In mks-5 mutants, most male tail cilia display PIP2 probe signal extending distally into the cilium. Scale bar: 4 μm.
Co-localisation of GFP-tagged PLC-δ-PH with MKSR-2 and TRAM-1 within phasmid cilia. MKSR-2 marks the TZ, and TRAM-1 marks the PCMC. In wild-type animals, the majority of the PIP2 probe signal localises to the PCMC and decreases in signal intensity distally into the cilium across the TZ. In mks-5 mutants, which lack an identifiable TZ, the PIP2 probe signal extends distally into the cilium. Shown in the graphs are the quantification of ten cilia for each strain indicated; error bars are median absolute deviation and dots indicate P-values (small: P < 0.05, medium: P < 0.01, large: P < 0.001, Kruskal–Wallis test). In the mks-5 mutant, PIP2 ectopic ciliary localisation is observed simultaneously with TRAM-1 leaking into the cilium, as well as MKSR-2 delocalisation, as previously reported (Williams et al, 2011). Scale bars: 4 μm.
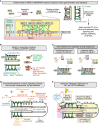
Summary depicting role of MKS-5 in forming a TZ that helps limit ciliary PIP2 concentration and compartmentalises signalling to the distal ciliary region. MKS and NPHP module components are shown in yellow and green, respectively, and MKS-5 in red. Inset shows organisational hierarchy of TZ proteins, with MKS-5 playing a central role in assembling two distinct modules (MKS and NPHP); arrows indicate requirement for particular TZ proteins in localising other(s) to the TZ (e.g. MKS-5 is required for correct NPHP-4 localisation), and white circles on arrowheads denote new functional interactions uncovered in this study.
Model to explain the redundant functions of the MKS and NPHP modules in basal body docking, formation of the TZ, and sealing of the ciliary compartment. MKS module components operate at the TZ, whereas NPHP module proteins act at the TZ and basal body–transition fibres. Loss of either module impairs the ciliary gate (Williams et al, 2011) but does not severely impact TZ formation or ciliogenesis; disruption of both affects the “seal” formed between the transition fibres/TZ and ciliary membrane.
Two models for the role of MKS-5 in TZ assembly during ciliogenesis. Different stages shown are separated by arrows and lead to a mature (fully formed) TZ at the bottom. Left: MKS-5 acts as a structural anchor (“scaffold”) for MKS/NPHP module components to build TZ ultrastructure (including Y-links) constructed from MKS and NPHP module components. In this model, MKS-5 is a scaffold at each TZ Y-link structure (green). Right: MKS-5 acts as an assembly factor (“chaperone”) in assembling MKS/NPHP module components. In this model, MKS-5 borders the forming TZ as it assists in the assembly of the Y-links using MKS/NPHP module components.
Distinct lipid domains help establish a CIZE at the TZ, and compartmentalise signalling proteins within the ciliary organelle. The phosphoinositide PI(4,5)P2 (PIP2) is enriched at the periciliary membrane and displays much reduced concentrations towards the distal ciliary region. Likely diffusion barriers are shown as orange double-headed arrows. The lipophilic dye DiI is excluded from the distal segment (DS) consisting of singlet microtubules (MTs), defining another lipid environment. The so-called inversin (inv) compartment, which contains inversin and other signalling proteins, represents the region between the two likely different lipid domains, at the TZ and DS.
Potential role of PIP2 in the trafficking of ciliary GPCRs. Interactions between GPCRs and IFT machinery (via TULP3; Mukhopadhyay et al, 2010) are stabilised by the high concentration of PIP2 at the periciliary membrane–basal body region (the site of IFT machinery assembly), and weakened distal to this domain, causing cargo release and retention within the cilium by the CIZE. The ciliary inositol polyphosphate 5-phosphatases INPP5E and OCRL1 may participate in reducing PIP2 concentrations by hydrolysing PIP2 to PI4P within the cilium. The anterograde IFT machinery is shown moving from its site of docking/assembly through the TZ and into the distal regions of the cilium (retrograde transport is not shown).
References
-
- Arts HH, Doherty D, van Beersum SE, Parisi MA, Letteboer SJ, Gorden NT, Peters TA, Marker T, Voesenek K, Kartono A, Ozyurek H, Farin FM, Kroes HY, Wolfrum U, Brunner HG, Cremers FP, Glass IA, Knoers NV, Roepman R. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet. 2007;39:882–888. - PubMed
-
- Baker K, Beales PL. Making sense of cilia in disease: the human ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151C:281–295. - PubMed
-
- Bednarek EM, Schaheen L, Gaubatz J, Jorgensen EM, Fares H. The plasma membrane calcium ATPase MCA-3 is required for clathrin-mediated endocytosis in scavenger cells of Caenorhabditis elegans. Traffic. 2007;8:543–553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

