Polymorphs of Theophylline Characterized by DNP Enhanced Solid-State NMR
- PMID: 26393368
- PMCID: PMC4699642
- DOI: 10.1021/acs.molpharmaceut.5b00610
Polymorphs of Theophylline Characterized by DNP Enhanced Solid-State NMR
Abstract
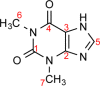
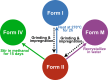
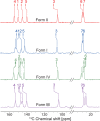
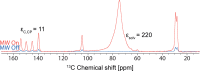
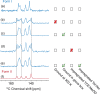
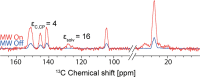
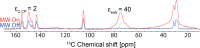
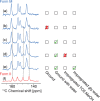
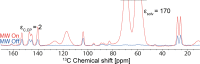
We show how dynamic nuclear polarization (DNP) enhanced solid-state NMR spectroscopy can be used to characterize polymorphs and solvates of organic solids. We applied DNP to three polymorphs and one hydrated form of the asthma drug molecule theophylline. For some forms of theophylline, sample grinding and impregnation with the radical-containing solution, which are necessary to prepare the samples for DNP, were found to induce polymorphic transitions or desolvation between some forms. We present protocols for sample preparation for solid-state magic-angle spinning (MAS) DNP experiments that avoid the polymorphic phase transitions in theophylline. These protocols include cryogrinding, grinding under inert atmosphere, and the appropriate choice of the impregnating liquid. By applying these procedures, we subsequently demonstrate that two-dimensional correlation experiments, such as (1)H-(13)C and (1)H-(15)N HETCOR or (13)C-(13)C INADEQUATE, can be obtained at natural isotopic abundance in reasonable times, thus enabling more advanced structural characterization of polymorphs.
Keywords: dynamic nuclear polarization; polymorphs; solid-state NMR; theophylline.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Bernstein J.Polymorphism in Molecular Crystals; Oxford University Press: Oxford, 2002.
-
- Polymorphism, 1st ed.; Wiley-VCH: Weinheim, 2006.
-
- Day G. M. Current approaches to predicting molecular organic crystal structures. Crystallogr. Rev. 2011, 17, 3–52. 10.1080/0889311X.2010.517526. - DOI
-
- Vasileiadis M.; Pantelides C. C.; Adjiman C. S. Prediction of the crystal structures of axitinib, a polymorphic pharmaceutical molecule. Chem. Eng. Sci. 2015, 121, 60–76. 10.1016/j.ces.2014.08.058. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

