Rivastigmine for Alzheimer's disease
- PMID: 26393402
- PMCID: PMC7050299
- DOI: 10.1002/14651858.CD001191.pub4
Rivastigmine for Alzheimer's disease
Abstract
Background: Alzheimer's disease is the commonest cause of dementia affecting older people. One of the therapeutic strategies aimed at ameliorating the clinical manifestations of Alzheimer's disease is to enhance cholinergic neurotransmission in the brain by the use of cholinesterase inhibitors to delay the breakdown of acetylcholine released into synaptic clefts. Tacrine, the first of the cholinesterase inhibitors to undergo extensive trials for this purpose, was associated with significant adverse effects including hepatotoxicity. Other cholinesterase inhibitors, including rivastigmine, with superior properties in terms of specificity of action and lower risk of adverse effects have since been introduced. Rivastigmine has received approval for use in 60 countries including all member states of the European Union and the USA.
Objectives: To determine the clinical efficacy and safety of rivastigmine for patients with dementia of Alzheimer's type.
Search methods: We searched ALOIS, the Cochrane Dementia and Cognitive Improvement Group Specialized Register, on 2 March 2015 using the terms: Rivastigmine OR exelon OR ENA OR "SDZ ENA 713". ALOIS contains records of clinical trials identified from monthly searches of a number of major healthcare databases (Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL, LILACS), numerous trial registries and grey literature sources.
Selection criteria: We included all unconfounded, double-blind, randomised, controlled trials in which treatment with rivastigmine was administered to patients with dementia of the Alzheimer's type for 12 weeks or more and its effects compared with those of placebo in a parallel group of patients, or where two formulations of rivastigmine were compared.
Data collection and analysis: One review author (JSB) applied the study selection criteria, assessed the quality of studies and extracted data.
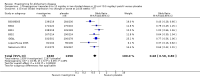
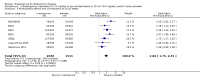
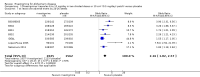
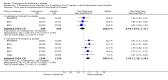
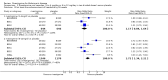
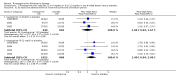
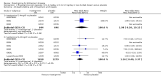
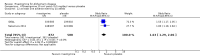
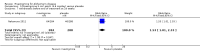
Main results: A total of 13 trials met the inclusion criteria of the review. The trials had a duration of between 12 and 52 weeks. The older trials tested a capsule form with a dose of up to 12 mg/day. Trials reported since 2007 have tested continuous dose transdermal patch formulations delivering 4.6, 9.5 and 17.7 mg/day.Our main analysis compared the safety and efficacy of rivastigmine 6 to 12 mg/day orally or 9.5 mg/day transdermally with placebo.Seven trials contributed data from 3450 patients to this analysis. Data from another two studies were not included because of a lack of information and methodological concerns. All the included trials were multicentre trials and recruited patients with mild to moderate Alzheimer's disease with a mean age of about 75 years. All had low risk of bias for randomisation and allocation but the risk of bias due to attrition was unclear in four studies, low in one study and high in two studies.After 26 weeks of treatment rivastigmine compared to placebo was associated with better outcomes for cognitive function measured with the Alzheimer's Disease Assessment Scale-Cognitive (ADAS-Cog) score (mean difference (MD) -1.79; 95% confidence interval (CI) -2.21 to -1.37, n = 3232, 6 studies) and the Mini-Mental State Examination (MMSE) score (MD 0.74; 95% CI 0.52 to 0.97, n = 3205, 6 studies), activities of daily living (SMD 0.20; 95% CI 0.13 to 0.27, n = 3230, 6 studies) and clinician rated global impression of changes, with a smaller proportion of patients treated with rivastigmine experiencing no change or a deterioration (OR 0.68; 95% CI 0.58 to 0.80, n = 3338, 7 studies).Three studies reported behavioural change, and there were no differences compared to placebo (standardised mean difference (SMD) -0.04; 95% CI -0.14 to 0.06, n = 1529, 3 studies). Only one study measured the impact on caregivers using the Neuropsychiatric Inventory-Caregiver Distress (NPI-D) scale and this found no difference between the groups (MD 0.10; 95% CI -0.91 to 1.11, n = 529, 1 study). Overall, participants who received rivastigmine were about twice as likely to withdraw from the trials (odds ratio (OR) 2.01, 95% CI 1.71 to 2.37, n = 3569, 7 studies) or to experience an adverse event during the trials (OR 2.16, 95% CI 1.82 to 2.57, n = 3587, 7 studies).
Authors' conclusions: Rivastigmine (6 to 12 mg daily orally or 9.5 mg daily transdermally) appears to be beneficial for people with mild to moderate Alzheimer's disease. In comparisons with placebo, better outcomes were observed for rate of decline of cognitive function and activities of daily living, although the effects were small and of uncertain clinical importance. There was also a benefit from rivastigmine on the outcome of clinician's global assessment. There were no differences between the rivastigmine group and placebo group in behavioural change or impact on carers. At these doses the transdermal patch may have fewer side effects than the capsules but has comparable efficacy. The quality of evidence is only moderate for all of the outcomes reviewed because of a risk of bias due to dropouts. All the studies with usable data were industry funded or sponsored. This review has not examined economic data.
Conflict of interest statement
None known
Figures
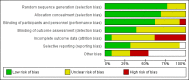












































































































































Update of
References
References to studies included in this review
B103 {published data only}
-
- Agid Y, Dubois B on behalf of the International Rivastigmine Investigators, Anand R, Gharabawi G. Efficacy and tolerability of rivastigmine in patients with dementia of the Alzheimer type. Current Therapeutic Research 1998;59(12):837‐45.
-
- Anand R, Gharabawi G, Enz A. Efficacy and safety results of the early phase studies with Exelon (ENA‐713) in Alzheimer's Disease: an overview. Journal of Drug Development and Clinical Practice 1996;8(2):109‐16.
B104 {published data only}
-
- Anand R, Gharabawi G, Enz A. Efficacy and safety results of the early phase studies with Exelon (ENA‐713) in Alzheimer's Disease: an overview. Journal of Drug Development and Clinical Practice 1996;8(2):109‐16.
-
- Forette F, Anand R, Gharabawi G. A phase II study in patients with Alzheimer's disease to assess the preliminary efficacy and maximum tolerated dose of rivastigmine (Exelon®). European Journal of Neurology 1999;6:423‐9. - PubMed
B303/B305 {published and unpublished data}
-
- Anand R, Hartman R, Graham S. Effects of Alzheimer's disease severity on activities of daily living with long‐term rivastigmine treatment. Journal of the American Geriatrics Society 2001;49(4):S151.
-
- Anand R, Messina J, Veach J, Hartman R. Effects of rivastigmine in patients with moderately severe Alzheimer's disease. Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; April 5‐8, 2000; Stockholm, Sweden. 2000:199.
-
- Burns A, Spiegel R, Quarg P. Efficacy of rivastigmine in subjects with moderately severe Alzheimer's disease. International Journal of Geriatric Psychiatry 2004;19(3):243‐9. - PubMed
-
- Erkinjuntti T, Skoog I, Lane R, Andrews C. Potential long‐term effects of rivastigmine on disease progression may be linked to drug effects on vascular changes in Alzheimer brains. International Journal of Clinical Practice 2003;57(9):756‐60. - PubMed
-
- Erkinjuntti T, Skoog I, Lane R, Andrews C. Rivastigmine in patients with Alzheimer's disease and concurrent hypertension. International Journal of Clinical Practice 2002;56(10):791‐6. - PubMed
B304 {published and unpublished data}
-
- Lindesay J. An open label one‐year extension of SDZ‐ENA‐713 studies B303, B304 and B305 to prospectively evaluate long‐term safety, tolerability and efficacy of SDZ‐ENA‐713 in out‐patients with probable Alzheimer's disease. National Research Register 2000.
-
- Novartis. No title. Unpublished. Data provided by Novartis. No year.
-
- Novartis Pharmaceuticals. ADENA Programme. Unpublished Data 1998.
B351 {unpublished data only}
-
- Burns A, Spiegel R, Quarg P. Efficacy of rivastigmine in subjects with moderately severe Alzheimer's disease. International Journal of Geriatric Psychiatry 2004;19(3):243‐9. - PubMed
-
- Farlow M, Potkin S, Koumaras B, Veach J, Mirski D. Analysis of outcome in retrieved dropout patients in a rivastigmine vs placebo, 26‐week, Alzheimer disease trial. Archives of Neurology 2003;60:843‐8. - PubMed
-
- Novartis. No title. Unpublished. Data provided by Novartis. No year.
-
- Novartis Pharmaceuticals. ADENA Programme. Unpublished Data 1998.
B352 {published data only}
-
- Burns A, Spiegel R, Quarg P. Efficacy of rivastigmine in subjects with moderately severe Alzheimer's disease. International Journal of Geriatric Psychiatry 2004;19(3):243‐9. - PubMed
-
- Corey‐Bloom J, Anand R, Veach J for ENA 713 B352 Study Group. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer's disease. International Journal of Geriatric Psychopharmacology 1998;1:55‐65.
-
- Doraiswamy M. The effects of rivastigmine on the Alzheimer's disease assessment scale‐cognitive subscale items scores of patients with Alzheimer's disease. Health in aging the challenge and promise of new decade. Proceedings of the annual scientific meeting of the American Geriatrics Society and the American Federation for Aging Research; 2000 May 17‐21, Nashville. 2000:173.
-
- Doraiswamy PM, Anand R, Hartman R. Cognitive effects of rivastigmine in patients with mild to moderate alzheimer's disease compared to those with moderately‐severe to severe AD. Clinical Neuropschological Assessment 2000;1(6):12.
-
- Doraiswamy PM, Anand R, Hartman R. Long term cognitive effects in Alzheimer's disease patients stratified by vascular risk score and treated for 1 year with rivastigmine. Clinical Neuropschological Assessment 2000;1(6):14.
Ballard 2005 {published data only}
IDEAL {published data only}
-
- Alva G, Grossberg G, Schmitt F, Olin J. Influence of rivastigmine on activities of daily living: item responder analyses targeting improvement and atability. Annals of Neurology 2009; Vol. 66:S49.
-
- Alva G, Grossberg GT, Schmitt FA, Meng X, Olin JT. Efficacy of rivastigmine transdermal patch on activities of daily living: item responder analyses. International Journal of Geriatric Psychiatry 2011; Vol. 26, issue 4:356‐63. - PubMed
-
- Blesa R, Ballard C, Orgogozo JM, Lane R, Thomas SK. Caregiver preference for rivastigmine patches versus capsules for the treatment of Alzheimer's disease. Neurology 2007;69(4 Suppl 1):S23‐8. - PubMed
-
- Cummings J, Winblad B. A rivastigmine patch for the treatment of Alzheimer's disease and Parkinson's disease dementia. Expert Review on Neurotherapeutics 2007;7(11):1457‐63. - PubMed
-
- Cummings JL, Farlow MR, Meng X, Tekin S, Olin JT. Rivastigmine transdermal patch skin tolerability: results of a 1‐year clinical trial in patients with mild‐to‐moderate Alzheimer's disease. Clinical Drug Investigation 2010; Vol. 30, issue 1:41‐9. - PubMed
Karaman 2005 {published data only}
-
- Karaman Y, Erdogan F, Koseoglu E, Turan T, Ersoy AO. A 12‐month study of the efficacy of rivastigmine in patients with advanced moderate Alzheimer's disease. Dementia and Geriatric Cognitive Disorders 2005;19(1):51‐6. - PubMed
Lopez‐Pousa 2005 {published data only}
-
- Lopez‐Pousa S. Pilot, multicenter, randomized, double‐blind, controlled, parallel efficacy and safety study of rivastigmine vs placebo in the treatment of cognitive and non‐cognitive symptoms in patients with moderate‐to‐severe Alzheimer's disease. IFPMA Register 2005.
Mowla 2007 {published data only}
-
- Mowla A, Mosavinasab M, Haghshenas H, Haghighi AB. Does serotonin augmentation have any effect on cognition and activities of daily living in Alzheimer's dementia? A double‐blind, placebo‐controlled clinical trial. Journal of Clinical Psychopharmacology 2007;27(5):484‐7. - PubMed
Nakamura 2011 {published data only}
-
- Nakamura Y, Imai Y, Shigeta M, Graf A, Shirahase T, Kim H, et al. A 24‐week, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy, safety and tolerability of the rivastigmine patch in Japanese patients with Alzheimer's disease. Dementia and Geriatric Cognitive Disorders Extra 2011; Vol. 1, issue 1:163‐79. - PMC - PubMed
Tai 2000 {published data only}
-
- Tai CT, Liu CK, Sung SM, Pai MC, Hsu CY. The safety and efficacy of Exelon in Alzheimer's patients: A multicentre, randomized, 26‐week study in Taiwan. International Journal of Neuropsychopharmacology 2000;3 Suppl 1:S356. [MEDLINE: ]
References to studies excluded from this review
ACTION {published data only}
-
- Alva G, Cummings J, Galvin J, Meng X, Somogyi M. Infrequent skin reactions at the application site of the rivastigmine patch (4.6, 9.5 or 13.3 mg/24 h): Analysis of two clinical studies revealed most were tolerable and manageable across all doses. Alzheimer's & Dementia 2013;Conference: Alzheimer's Association International Conference 2013 Boston, MA United States. Conference Start: 20130713 Conference End: 20130718. Conference Publication:(var.pagings):P666.
-
- Farlow M, Grossberg G, Sadowsky C, Meng X, Somgyi M. Long‐term safety and efficacy of 13.3 mg/24 h rivastigmine patch in severe Alzheimer's disease: ACTivities of daily living and cognitION (ACTION) study. Journal of the Neurological Sciences 2013;Conference: 21st World Congress of Neurology Vienna Austria. Conference Start: 20130921 Conference End: 20130926. Conference Publication:(var.pagings):e339.
-
- Farlow M, Grossberg G, Sadowsky C, Meng X, Somogyi M. A 24‐week, open‐label extension to the activities of daily living and cognition (ACTION) study: Long‐term safety, tolerability and efficacy of a 13.3 mg/24h rivastigmine patch in people with severe Alzheimer's disease. Alzheimer's & Dementia 2013;Conference: Alzheimer's Association International Conference 2013 Boston, MA United States. Conference Start: 20130713 Conference End: 20130718. Conference Publication:(var.pagings):P655.
-
- Farlow M, Grossberg G, Sadowsky C, Meng X, Somogyi M. Long‐term safety, tolerability and efficacy of 13.3 mg/24 h rivastigmine patch in patients with severe Alzheimer's disease. Clinical Pharmacology in Drug Development 2013;Conference: 2013 American College of Clinical Pharmacology Annual Meeting Bethesda, MD United States. Conference Start: 20130922 Conference End: 20130924. Conference Publication:(var.pagings):43.
-
- Farlow M, Meng X, Somogyi M. Efficacy, safety and tolerability of rivastigmine patch 13.3 mg/24 h (15 cm2) versus 4.6 mg/24 h (5 cm2) in patients with severe Alzheimer's disease: Results of the activities of daily living and cognition (ACTION) study. American journal of geriatric psychiatry 2013;Conference: 2013 AAGP Annual Meeting Los Angeles, CA United States. Conference Start: 20130314 Conference End: 20130317(var.pagings):S139‐40.
Almkvist 2004 {published data only}
-
- Almkvist O, Darreh Shori T, Stefanova E, Spiegel R, Nordberg A. Preserved cognitive function after 12 months of treatment with rivastigmine in mild Alzheimer's disease in comparison with untreated AD and MCI patients. European Journal of Neurology 2004;11(4):253‐61. - PubMed
Auriacombe 2002 {published data only}
-
- Auriacombe S, Pere JJ. No donepezil discontinuation effect in patients with Alzheimer's disease who were switched to rivastigmine after failing to benefit from donepezil treatment. Current Medical Research and Opinion 2003;19(8):715‐7. - PubMed
-
- Auriacombe S, Pere JJ, Loria Kanza, Vellas B. Efficacy and safety of rivastigmine in patients with Alzheimer's disease who failed to benefit from treatment with donepezil. Current Medical Research and Opinion 2002;18(3):129‐38. - PubMed
B105 {published data only}
-
- Anand R, Gharabawi G, Enz A. Efficacy and safety results of the early phase studies with Exelon (ENA‐713) in Alzheimer's disease: an overview. Journal of Drug Development and Clinical Practice 1996;8(2):109‐16.
-
- Cutler N, Sramek J, Anand R. Safety and tolerance of ENA 713 in Alzheimer's disease (AD). Proceedings of the 8th European College of Neuropsychopharmacology Congress (ECNP); 1995 Sep 30‐Oct 4, Venice. 1995. [MEDLINE: ]
-
- Cutler NR, Sramek JJ, Anand R. Safety and tolerance of ENA 713 in patients with Alzheimer's disease. Biological Psychiatry 1995;37(9):643.
-
- Sramek J, Anand R, Wardle T, Irwin P, Hartman R, Cutler N. Safety/tolerability trial of SDZ ENA 713 in patients with probable Alzheimer's disease. Life Sciences 1996;58(15):1201‐7. - PubMed
Bilikiewicz 2002 {published data only}
-
- Bilikiewicz A, Opala G, Podemski R, Puzynski S, Lapin J, Soltys K, et al. An open‐label study to evaluate the safety, tolerability and efficacy of rivastigmine in patients with mild to moderate probable Alzheimer's disease in the community setting. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 2002;8(2):PI9‐15. - PubMed
Blesa Gonzalez 2011 {published data only}
-
- Blesa Gonzalez R, Boada Rovira M, Martinez Parra C, Gil‐Saladie D, Almagro CA, Gobartt Vazquez AL, et al. Evaluation of the convenience of changing the rivastigmine administration route in patients with Alzheimer disease. Neurologia 2011; Vol. 26, issue 5:262‐71. - PubMed
Brassen 2003 {published data only}
-
- Brassen S, Adler G. Short‐term effects of acetylcholinesterase inhibitor treatment on EEG and memory performance in Alzheimer patients: an open, controlled trial. Pharmacopsychiatry 2003;36(6):304‐8. - PubMed
Caffarra 2007 {published data only}
-
- Caffarra P, Vezzadini G, Copelli S, Dieci F, Messa G, Nonis E, Venneri A. Comparing treatment effects in a clinical sample of patients with probable Alzheimer's disease treated with two different cholinesterase inhibitors. Acta Biomedica 2007;78(1):16‐21. - PubMed
Cummings 2000 {published data only}
-
- Aupperle PM, Koumaras B, Chen M, Rabinowicz A, Mirski D. Long‐term effects of rivastigmine treatment on neuropsychiatric and behavioral disturbances in nursing home residents with moderate to severe Alzheimer's disease: results of a 52‐week open‐label study. Current Medical Research and Opinion 2004;20(10):1605‐12. - PubMed
-
- Cummings J, Anand R, Koumaras, et al. Abstract S79.002. Neurology 2000;54:A468.
Cutler 1998 {published data only}
-
- Cutler NR, Polinsky R, Sramek JJ, Enz A, Jhee S, Mancione L, et al. Dose‐dependent CSF acetylcholinesterase inhibition by SDZ ENA 713 in Alzheimer's disease. Acta Neurologica Scandinavica 1998;97:244‐50. - PubMed
Cutler 2000 {published data only}
-
- Cutler NR, Hossain M, McDonald C, Pommier F, Sedek G, Jhee SS, Sramek JJ. Pharmacokinetics of rivastigmine and its metabolite in patients with Alzheimer's disease. Biological Psychiatry 2000;47 Suppl 1:S161.
-
- Hossain M, Jhee SS, Shiovitz T, McDonald C, Sedek G, Pommier F, Cutler NR. Non‐blinded bioavailability study of oral and intravenous rivastigmine. Clinical Pharmacokinetics 2002;41(3):225‐34. - PubMed
Dantoine 2006 {published data only}
-
- Dantoine T, Auriacombe S, Sarazin M, Becker H, PereJJ, Bourdeix I. Rivastigmine monotherapy and combination therapy with memantine in patients with moderately severe Alzheimer's disease who failed to benefit from previous cholinesterase inhibitor treatment. International Journal of Clinical Practice 2006;60(1):110‐8. - PubMed
Doraiswamy 2000a {published data only}
-
- Doraiswamy M. Early intervention with a cholinesterase inhibitor produces long‐term beneficial effects in moderately severe ad patients. Proceedings of the World Alzheimer Congress; 2000 Jul 9‐13, Washington. 2000a.
Edwards 2002 {published data only}
-
- Edwards K, Goddman W, Anand R, Koumars B, Hartman R. Effect of Alzheimer's disease severity on psychotropic drug use and behavior in nursing home patients treated with rivastigmine. The 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:296.
EXCEED {published data only}
-
- Anon. Double‐blind trial will compare two anti‐Alzheimer's drugs. Journal of Dementia Care 2001c; Vol. 9, issue 5:6.
-
- Blesa R, Bullock R, He Y, Bergman H, Gambina G, Meyer J, et al. Effect of butyrylcholinesterase genotype on the response to rivastigmine or donepezil in younger patients with Alzheimer's disease. Pharmacogenetic Genomics 2006;16(11):771‐4. - PubMed
-
- Bullock R, Bergman H, Touchon J, Gasmbina G, He Y, Nagel J, Lane R. Effect of age on response to rivastigmine or donepezil in patients with Alzheimer's disease. Current Medical Research and Opinion 2006;22(3):483‐94. - PubMed
-
- Bullock R, Touchon J, Bergman H, Gambina G, He Y, Rapatz G, et al. Rivastigmine and donepezil treatment in moderate to moderately severe Alzheimer's disease over a 2‐year period. Current Medical Research and Opinion 2005;21(8):1317‐27. - PubMed
-
- Bullock R, Touchon J, Bergman H, Gambina G, He Y, Rapatz G, et al. Rivastigmine and donepezil treatment in moderate to moderately‐severe Alzheimer's disease over a 2‐year period. Current Medical Research and Opinion 2005;21(8):1317‐28. - PubMed
Frankfort 2007 {published data only}
-
- Frankfort SV, Appels BA, Boer A, Tulner LR, Campen JPCM, Koks CHW, et al. Identification of responders and reactive domains to rivastigmine in Alzheimer's disease. Pharmacoepidemiology and Drug Safety 2007;16(5):545‐51. - PubMed
Fuschillo 2001 {published data only}
-
- Fuschillo C, Pia S, Campana F, Pinto A, Simone L. Cognitive deficits in Alzheimer's disease: treatment with acetylcholinesterase inhibitor agents. Archives of Gerontology and Geriatrics 2001;33 Suppl 1:151‐8. - PubMed
Holmes 2007 {published data only}
-
- Holmes C, Wilkinson D, Dean C, Clare C, El‐Okl M, Hensford C, Moghul S. Risperidone and rivastigmine and agitated behaviour in severe Alzheimer's disease: a randomised double blind placebo controlled study. International Journal of Geriatric Psychiatry 2007;22(4):380‐1. - PubMed
InDDEx {published data only}
-
- Feldman H, Scheltens E, Hermann N, Ferris S, Mesenbrink P, Satlin A, Mancione L. Behavioral symptoms in mild cognitive impairment findings from the InDDEx study. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:522.
-
- Feldman H, Scheltens P, Scarpini E, Hermann N, Mesenbrink P, Mancione L, et al. Behavioural symptoms in mild cognitive impairment. Neurology 2004;62:1199‐201. - PubMed
-
- Ferris S. Investigation into Delay to Diagnosis of Alzheimer's Disease with Exelon (InDDEx). Alzheimer's Disease Education and Referral Center (ADEAR) 1999a. [MEDLINE: ]
-
- Ferris S, Feldman P, Mesenbrink P, Mancione L, Satlin A. Mild Cognitive impairment and Alzheimer's disease are these distinct study populations clinical trials. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:570.
-
- Rossor MN. A prospective randomised mc double blind placebo controlled parallel group study of the effect of Exelon on the time to clinical diagnosis of Alzheimer's disease in subjects with mild cognitive impairment. Current Controlled Trials 2001.
Kim 2002 {published data only}
-
- Kim SY. A 24 week trial investigating the safety tolerability and efficacy of rivastigmine in mild to moderately severe Alzheimer's disease patients in Korea. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:360.
Malsch 2001 {published data only}
-
- Malsch U, Dennler HJ. [Treatment of Alzheimer's disease: Tolerability of rivastigmine during the initial period]. Psychopharmacology 2001;27(6):337‐42.
McMillan 1999 {published data only}
-
- McMillan H. Drug treatment of Alzheimer's disease and responders to rivastigmine beyond 12 weeks [letter]. International Journal of Geriatric Psychiatry 1999; Vol. 14, issue 12:1078‐9. - PubMed
Novartis 2005 {published data only}
-
- Novartis Pharmaceuticals Corporation. An open‐label extension to evaluate the efficacy and safety of the rivastigmine transdermal patch in patients with probable Alzheimer's sisease. ClinicalTrials.gov 2005.
OPTIMA {published data only}
-
- Alva G, Isaacson R, Sadowsky C, Grossberg G, Meng X, Somogyi M. Efficacy of higher‐dose 13.3 mg/24 h (15cm2) rivastigmine patch on the Alzheimer's Disease Assessment Scale‐cognitive subscale: domain and individual item analysis. International Journal of Geriatric Psychiatry 2014;29(9):920‐7. - PubMed
-
- Black S, Bakchine S, Bellelli G, Molinuevo JL, Downs P, Caputo A, et al. Efficacy of the 13.3 MG/24 h rivastigmine patchoninstrumentalactivitiesofdaily living in the optimising transdermal exelon in mild‐to‐moderate Alzheimer's disease (optima) study: Prospective subgroup analysis by disease severity and time‐to‐meet decline. Alzheimer's and Dementia 2012; Vol. Conference: Alzheimer's Association International Conference 2012 Vancouver, BC Canada. Conference Start: 20120714 Conference End: 20120719. Conference Publication:, issue var.pagings.
-
- Blesa R, Martinez‐Lage P, Monsch AU, Downs P, Strohmaier C. Caregiver preference for rivastigmine patch in the OPtimising Transdermal exelon In Mild‐to‐moderate Alzheimer's disease (OPTIMA) Study. European Journal of Neurology 2012; Vol. Conference: 16th Congress of the European Federation of Neurological Societies, EFNS Stockholm Sweden. Conference Start: 20120908 Conference End: 20120911. Conference Publication:, issue var.pagings.
-
- Cummings J, Bellelli G, Black S, Bakchine S, Krahnke T, Strohmaier C. The rivastigmine high‐dose, 13 mg/24h (15cm2), transdermal patch provides daily living benefits to people with severe Alzheimer's disease: Retrospective analyses of the optimising transdermal exelon in mild‐to‐moderate Alzheimer's disease (OPTIMA) study. Alzheimer's & Dementia 2013;Conference: Alzheimer's Association International Conference 2013 Boston, MA United States. Conference Start: 20130713 Conference End: 20130718. Conference Publication:(var.pagings):P658.
-
- Cummings J, Froelich L, Black SE, Bakchine S, Bellelli G, Molinuevo J, et al. Randomized, double‐blind, parallel‐group, 48‐week study for efficacy and safety of a higher‐dose rivastigmine patch (15 vs. 10 cm) in Alzheimer's disease. Dementia and Geriatric Cognitive Disorders 2012; Vol. 33, issue 5:341‐53. - PubMed
Potkin 1999a {published data only}
-
- Potkin SG, Anand R, Fleming K, Alva G, Keator D, Carreon D, et al. Brain metabolic and clinical effects of rivastigmine in Alzheimer's disease. International Journal of Neuropsychopharmacology 2001;4(3):223‐30. - PubMed
-
- Potkin SG, Wu JC, Messina J, Fleming K, Keator D, Bunney WE, An R. Neuroimaging techniques and applications. Proceedings of the 152nd Annual Meeting of the American Psychiatric Association; 1999 May 15‐20, Washington DC. 1999. [MEDLINE: ]
Riepe 2005 {published data only}
-
- Riepe MW, Adler G, Ibach B, Weinkauf B, Tracik F. Adding memantine to therapy with rivastigmine in patients with mild to moderate Alzheimer's disease: results of a 12‐week pilot study. 57th Annual Meeting of the American Academy of Neurology, Miami Beach, April 2005. 2005, issue P06.081.
Rozzini 2002 {published data only}
-
- Rozzini L, Bargnani C, Bosio A, Chia F, Franzani S, Leonardi R, et al. Acetylcholinesterase inhibitors are effective in real world patients with mild to moderate Alzheimer disease evidence from a large population treated with rivastigmine or donepezil. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:329.
-
- Rozzini L, Bargnani C, Bosio A, Chia F, Franzoni S, Leonardi R, et al. Comparison of efficacy and safety of rivastigmine and donepezil in patients with mild to moderate Alzheimer disease: results from a multicentre randomised trial. Proceedings of the 7th International Geneva/Springfield Symposium on Advances in Alzheimer Therapy, 2002 Apr 3‐6, Geneva. 2002:240.
Schmidt 2002 {published data only}
-
- Schmidt R, Lechner A, Petrovic K. Rivastigmine in outpatient services: experience of 114 neurologists in Austria. International Clinical Psychopharmacology 2002;17(2):81‐5. - PubMed
Shanks 2001 {published data only}
-
- Shanks MF, Venneri A, Staff RT, Pestell SJ, Forbes KE, Gemmell HG, Murray AD. Cerebral blood flow and cognition enhanced by rivastigmine in a controlled study of Alzheimer's disease. Alzheimer's Disease International, 17th International Conference, Christchurch, New Zealand, 25‐27 October 2001. 2001:37.
Shua‐Haim 2002a {published data only}
-
- Shua‐Haim J, Smith J, Potel S. A head to study of donepezil (Aricept), rivastigmine (Exelon) and galantamine (Reminyl) for the treatment of Alzheimer's disease safety tolerability clinical and caregiver impression after 4‐5 months of treatment: a prospective study. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:286.
Shua‐Haim 2002b {published data only}
-
- Shua‐Haim J, Smith J, Amin S, Shua‐Haim V. Comparison of combination therapy with rivastigmine exelon and donepezil aricept versus rivastigmine alone for treatment of Alzheimer's disease safety tolerability and clinical experience after one year of treatment a cross section study. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:292.
Shua‐Haim 2002c {published data only}
-
- Shua‐Haim JR, Smith JM, Amin S. Slow dose escalation of rivastigmine (Exelon®) treatment of agitation in patients with alzheimer disease: an eight month prospective study. The International Symposium on Advances in Alzheimer Therapy, 2002, Geneva. 2002:242.
Small 2005 {published data only}
-
- Small GW, Kaufer D, Mendiondo MS, Quarg P, Spiegel R. Cognitive performance in Alzheimer's disease patients receiving rivastigmine for up to 5 years. International Journal of Clinical Practice 2005;59(4):473‐7. - PubMed
Sobow 2002 {published data only}
-
- Sobow T. Cholinesterase inhibitors in the real world: donepezil vs rivastigmine tolerability study. Proceedings of the 7th International Geneva/Springfield Symposium on Advances in Alzheimer Therapy, 2002 Apr 3‐6, Geneva. 2002:244.
Stefanova 2002 {published data only}
-
- Stefanova E, Blennow K, Darreh‐Shori T, Hellstrom‐Lindhal E, Wall A, Almkvist O, et al. Evaluation of cerebral glucose metabolism, CSF‐TAU and CSF‐A342 after long‐term rivastigmine and tacrine treatments in Alzheimer disease patients. Proceedings of the 7th International Geneva/Springfield Symposium on Advances in Alzheimer Therapy, 2002 Apr 3‐6, Geneva. 2002:246.
Thomas 2001 {published data only}
-
- Thomas A, Iacono D, Bonanni L, D' Andreamatteo G, Onofrj M. Donepezil, rivastigmine,and vitamin E in Alzheimer disease: A combined P300 event‐related potentials/neuropsychologic evaluation over 6 months. Clinical Neuropharmacology 2001;24(1):31‐42. - PubMed
Tsolaki 2002 {published data only}
-
- Tsolaki M, Gerothanassis D, Aristotle CP. Efficacy and safety of cholinesterase inhibitors a longitudinal comparative study between donepezil and rivastigmine. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:2038.
Venneri 2002 {published data only}
-
- Venneri A, Shanks MF. Charting patterns of progression in treated and untreated patients with Alzheimer's disease using SPECT. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:474.
Wang 2001 {published data only}
-
- Wang Y, Chen Q, Zhang Z, et al. The treatment by using rivastigmine for patients with alzheimer disease: results of a multicenter, randomized, open‐labeled, controlled clinical trial. Chinese Journal of Neurology 2001;34(4):210‐3.
Wang 2003 {published data only}
-
- Wang QH, Zhang ZX, Xu XH. [Efficacy of rivastigmine in treating mild to severe alzheimer's disease with different hachinski ischemia index]. Chinese Journal of Neurology 2003;36(1):44‐7.
Weiser 2002 {published data only}
-
- Weiser M, Davidson M, Rotmensch H, Korczyn A, Hartman R, Cicin‐Sain A, Anand R. A pilot randomised open label trial assessing safety and pharmacokinetic parameters of co administration of rivastigmine with risperidone in dementia patients with behavioral disturbances. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:633. - PubMed
-
- Weiser M, Rotmensch HH, Korczyn AD, Hartman R, Cicin Sain A, Anand R, Rivastigmine Risperidone Study Group. A pilot, randomized, open‐label trial assessing safety and pharmacokinetic parameters of co‐administration of rivastigmine with risperidone in dementia patients with behavioral disturbances. International Journal of Geriatric Psychiatry 2002;17(4):343‐6. - PubMed
Werber 2002 {published data only}
-
- Werber EA, Klein C, Rabey MJ. Evaluation of cholinergic treatment in demented by p300 evoked related potentials. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:442.
Wilkinson 2002 {published data only}
-
- Bullock R, Passmore F, Potocnik F, Hock C. The tolerability, ease of use and efficacy of donepezil and rivastigmine in Alzheimer's disease patients: a 12‐week, multinational, comparative study. Journal of the American Geriatrics Society 2001;49(4):S19.
-
- Potocnik FC, Smith R, Passmore P, Hock C, Wilkinson D, Maud CM, Hopker S. Tolerability, ease of use, and efficacy of donepezil and rivastigmine in Alzheimer's disease patients. Proceedings of the Annual Meeting of the American Psychiatric Association; 2001 May 5‐10; New Orleans. 2001.
-
- Wilkinson D, Passmore P, Potocnik F, Maud C, Hock C. Donepezil compared to rivastigmine in Alzheimer's disease: similar efficacy but better tolerability and physician and caregiver satisfaction in a multinational randomized trial. Proceedings of the 14th Annual Meeting of the American Association for Geriatric Psychiatry; 2001 Feb 23‐26, San Francisco. 2001.
-
- Wilkinson DG, Passmore AP, Bullock R, Hopker SW, Smith R, Potocnik FCV, et al. A multinational, randomised, 12‐week, comparative study of donepezil and rivastigmine in patients with mild to moderate Alzheimer's disease. International Journal of Clinical Practice 2002;56(6):441‐6. - PubMed
Additional references
Benton 1974
-
- Benton AL. Revised visual retention test. 4th Edition. San Antonio, Texas: The Psychological Association, 1974.
Birks 2006
-
- Birks JS, Harvey R. Donepezil for dementia due to Alzheimer's disease. Cochrane Database of Systematic Reviews 2006, Issue 1. [Art. No.: CD001190. DOI: 10.1002/14651858.CD001190] - PubMed
Birks 2008
-
- Birks J. The evidence for the efficacy of cholinesterase inhibitors in the treatment of Alzheimer's disease is convincing. International Psychogeriatrics 2008;20(2):279‐86. - PubMed
Blessed 1968
-
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and senile change in the cerebral grey matter of elderly subjects. British Journal of Psychiatry 1968;114:797‐811. - PubMed
Brunner 1990
-
- Brunner C, Spiegel R. Eine Validierungsstudie mit der NOSGER (Nurses' Observation Scale for Geriatric Patients), einem neuen Beurteilungsinstrument für die Psychogeriatrie [Eine Validierungsstudie mit der NOSGER (Nurses' Observation Scale for Geriatric Patients), einem neuen Beurteilungsinstrument für die Psychogeriatrie]. Zeitschr für Psychologie 1990;XIX(3):211‐9.
Burns 2004
-
- Burns A, Spiegel R, Quarg P. Efficacy of rivastigmine in subjects with moderately severe Alzheimer's disease. International Journal of Geriatric Psychiatry 2004;19(3):243‐9. - PubMed
Clegg 2002
-
- Clegg A, Bryant J, Nicholson T, McIntyre L, Broe S, Gerard K, Waugh N. Clinical and cost‐effectiveness of donepezil, rivastigmine, and galantamine for Alzheimer's disease: a systematic review. International Journal of Technology Assessment in Health Care 2002;18(3):497‐507. - PubMed
Cohen‐Mansfield 1995
-
- Cohen‐Mansfield J. Assessment of disruptive behaviour/agitation in the elderly: function, methods and difficulties. Journal of Geriatric Psychiatry Neurology 1995;8:52‐60. - PubMed
Cummings 1994
-
- Cummings JL, Mega M, Gray K, Rosenburg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308‐13. - PubMed
DeJong 1989
-
- DeJong R, Osterlund OW, Roy GW. Measurement of Quality‐of‐Life changes in patients with Alzheimer's Disease. Clinical Therapeutics 1989;11(4):545‐54. - PubMed
DSM IV
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington DC: American Psychiatric Press, 1994.
Erkinjuntti 2002
-
- Erkinjuntti T, Skoog I, Lane R, Andrews C. Rivastigmine in patients with Alzheimer's disease and concurrent hypertension. International Journal of Clinical Practice 2002;56:791‐6. - PubMed
Farlow 2003a
-
- Farlow M, Potkin S, Koumaras B, Veach J, Mirski D. Analysis of outcome in retrieved dropout patients in a rivastigmine vs placebo, 26‐week, Alzheimer disease trial. Archives of Neurology 2003a;60(6):843‐8. - PubMed
Fillit 2004
-
- Fillit H, Hill J. The economic benefits of acetylcholinesterase inhibitors for patients with alzheimer disease and associated dementias. Alzheimer Disease and Associated Disorders 2004;18 Suppl 1:S24‐9. - PubMed
Folstein 1975
-
- Folstein MF, Folstein SE, McHugh PR. ‘Mini‐Mental State’: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189‐98. - PubMed
Fuld 1981
-
- Fuld PA. The Fuld object memory evaluation. Chicago: Steeling Instrument Co., 1981.
Galasko 1997
-
- Galasko D, Schmitt FA, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Disease and Associated Disorders 1997;11:33‐9. - PubMed
Gelinas 1999
-
- Gelinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer's disease: the disability assessment for dementia. American Journal of Occupational Therapy 1999;53:471‐81. - PubMed
Grossberg 2000
-
- Grossberg GT, Stahelin HB, Messina JC, Anand R, Veach J. Lack of adverse pharmacodynamic drug interactions with rivastigmine and twenty‐two classes of medications. International Journal of Geriatric Psychiatry 2000;15:242‐7. - PubMed
Guy 1976
-
- Guy W (editor). ECDEU Assessment manual for psychopharmacology. Rockville: National Institute of Mental Health, 1976.
Guyatt 2008
Hauber 2000
-
- Hauber AB, Gnanasakthy A, Mauskopf JA. Savings in the cost of caring for patients with Alzheimer's disease in Canada: an analysis of treatment with rivastigmine. Clinical Therapeutics 2000;22(4):439‐51. - PubMed
Helsinki declaration
-
- Declaration of Helsinki. http://www.faseb.org/arvo/helsinki.htm.
Higgins 2011
-
- Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. In: Higgins JPT, Green S editor(s). [updated March 2011]. Available from www.cochrane‐handbook.org.: The Cochrane Collaboration, 2011.
Homma 1991
-
- Homma A, Niina R, Ishii T, Hasegawa K. Development of a new rating scale for dementia in the elderly: Mental Function Impairment Scale (MENFIS). Japanese Journal of Geriatric Psychiatry 1991;2:1217‐22.
Howard 2011
-
- Howard R, Phillips P, Johnson T, O’Brien J, Sheehan B, Lindesay J, et al. Determining the minimum clinically important differences for outcomes in the DOMINO trial. International Journal of Geriatric Psychiatry 2011;26(8):812‐7. - PubMed
Jann 2000
-
- Jann MW. Rivastigmine, a new‐generation cholinesterase inhibitor for the treatment of Alzheimer's disease. Pharmacotherapy 2000;20:1‐12. - PubMed
Kennedy 1999
-
- Kennedy JS, Polinsky RJ, Johnson B, Loosen P, Enz A, Laplanche R, et al. Preferential cerebrospinal fluid acetylcholinesterase inhibition by rivastigmine in humans. Journal of Clinical Psychopharmacology 1999;19:513‐21. - PubMed
McKhann 1984
-
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical Diagnosis of Alzheimer's Disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939‐44. - PubMed
Novartis 1998
-
- Novartis Pharmaceutical (UK) Limited. EXELON (rivastigmine) Beyond cognition: prolonging functional ability. Product monograph. EXELON (rivastigmine) Beyond cognition: prolonging functional ability. Product monograph. Frimley, 1998.
Panisset 1994
-
- Panisset M, Roudier M, Saxton J, Boller F. Severe impairment battery: a neurological test for severely demented patients. Archives of Neurology 1994;51:41‐5. - PubMed
Polinsky 1998
-
- Polinsky RJ. Clinical pharmacology of rivastigmine: A new‐generation acetylcholinesterase inhibitor for the treatment of Alzheimer's disease. Clinical Therapeutics 1998;20(4):634‐46. - PubMed
Reisberg 1982
-
- Reisberg B, Ferris SH, Leon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. American Journal of Psychiatry 1982;139:1136‐9. - PubMed
Reisberg 1989
-
- Reisberg B, Franssen E. Stage specific incidence of potentially remediable behavioral symptoms in aging and AD: a study of 120 patients using the BEHAVE‐AD. Bulletin of Clinical Neuroscience 1989;54:95‐112.
Reisberg 1994
-
- Reisberg B, Ferris SH. CIBIC‐plus interview guide. Unknown, 1994.
Reitan 1958
-
- Reitan RM. The validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills 1958;8:271‐6.
Rosen 1984
-
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. American Journal of Psychiatry 1984;41:356‐64. - PubMed
Saxton 1990
-
- Saxton J, McGonigle‐Gibson K, Swihart A, Miller M, Boller F. Assessment of severely impaired patients: description and validation of a new neuropsychological test battery. Psychological Assessment 1990;2:298‐303.
Schneider 1997
-
- Schneider LS, Olin JT, Doody RS, et al. Validity and reliability of the Alzheimer's Disease Cooperative Study‐Clinical Global Impression of Change. The Alzheimer's Disease Cooperative Study. Alzheimer Disease and Associated Disorders 1997;11 Suppl 2:S22‐S32. - PubMed
Schneider 1998
-
- Schneider LS, Anand R, Farlow MR. Systematic review of the efficacy of rivastigmine for patients with Alzheimer's disease. International Journal of Geriatric Psychopharmacology 1998;1:S26‐S34.
Schrag 2012
-
- Schrag A, Schott JM, Alzheimer's Disease Neuroimaging Initiative. Research paper: What is the clinically relevant change on the ADAS‐Cog?. Journal of Neurology, Neurosurgery, and Psychiatry 2012;83(2):171‐3. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. - PubMed
Spencer 1998
-
- Spencer CM, Noble S. Rivastigmine. A review of its use in Alzheimer's disease. Drugs & Aging 1998;13(5):391‐411. - PubMed
Watson 1993
-
- Watson YI, Arfken CL, Birge SJ. Clock completion: an objective screening test for dementia. Journal of the American Geriatric Society 1993;41:1235‐40. - PubMed
Wechsler 1987
-
- Wechsler D. Wechsler Memory Scale‐Revised (WMS‐R). San Antonio: Psychological Corporation Harcourt Brace Jovanovich Inc, 1987.
Williams 2003
-
- Williams BR, Nazarians A, Gill MA. A review of rivastigmine: a reversible cholinesterase inhibitor. Clinical Therapeutics 2003;25(6):1634‐53. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

