Molecular Heterogeneity Within the Clinical Diagnosis of Pericentral Retinal Degeneration
- PMID: 26393467
- PMCID: PMC4585343
- DOI: 10.1167/iovs.15-17174
Molecular Heterogeneity Within the Clinical Diagnosis of Pericentral Retinal Degeneration
Abstract
Purpose: To characterize in detail the phenotype and genotype of patients with pericentral retinal degeneration (PRD).
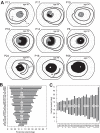
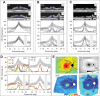
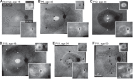
Methods: Patients were screened for an annular ring scotoma ranging from 3° to 40° (n = 28, ages 24-71) with kinetic perimetry. All patients had pigmentary retinopathy in the region of the dysfunction. Further studies included cross-sectional and en face imaging, static chromatic perimetry, and electroretinography. Molecular screening was performed.
Results: Genotypes of 14 of 28 PRD patients were identified: There were mutations in eight different genes previously associated with autosomal dominant or autosomal recessive RDs. Kinetic fields monitored in some patients over years to more than a decade could be stable or show increased extent of the scotoma. Electroretinograms were recordable but with different severities of dysfunction. Patterns of photoreceptor outer nuclear layer (ONL) loss corresponded to the distribution of visual dysfunction. Outer nuclear layer thickness topography and en face imaging indicated that the greatest disease expression was in the area of known highest rod photoreceptor density.
Conclusions: Molecular heterogeneity was a feature of the PRD phenotype. Many of the molecular causes were also associated with other phenotypes, such as maculopathies, typical retinitis pigmentosa (RP) and cone-rod dystrophy. The pericentral pattern of retinal degeneration is thus confirmed to be an uncommon phenotype of many different genotypes rather than a distinct disease entity.
Figures


References
-
- Grover S,, Fishman GA,, Brown J,, Jr. Patterns of visual field progression in patients with retinitis pigmentosa. Ophthalmology. 1998; 105: 1069–1075. - PubMed
-
- Gonin J. Le scotome annulaire dans la dégénérescence pigmentaire de la rétine. Ann d'Ocul. 1901; 125: 101–130.
-
- Francois J,, De Rouck A,, Cambie E,, De Laey JJ. Visual functions in pericentral and central pigmentary retinopathy. Ophthalmologica. 1972; 165: 38–61. - PubMed
-
- Krill AE. Central and Pericentral Retinitis Pigmentosa. Krill's Hereditary Retinal and Choroidal Diseases Volume 2. Hagerstown: Harper & Row Publishing Co.; 1977.
-
- Szamier RB,, Berson EL. Histopathologic study of an unusual form of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1982; 22: 559–570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

