Severe Nephrotoxic Nephritis following Conditional and Kidney-Specific Knockdown of Stanniocalcin-1
- PMID: 26393521
- PMCID: PMC4579070
- DOI: 10.1371/journal.pone.0138440
Severe Nephrotoxic Nephritis following Conditional and Kidney-Specific Knockdown of Stanniocalcin-1
Abstract
Background: Inflammation is the hallmark of nephrotoxic nephritis. Stanniocalcin-1 (STC1), a pro-survival factor, inhibits macrophages, stabilizes endothelial barrier function, and diminishes trans-endothelial migration of leukocytes; consistently, transgenic (Tg) overexpression of STC1 protects from nephrotoxic nephritis. Herein, we sought to determine the phenotype of nephrotoxic nephritis after conditional and kidney-specific knockdown of STC1.
Methods: We used Tg mice that, express either STC1 shRNA (70% knockdown of STC1 within 4d) or scrambled shRNA (control) upon delivery of Cre-expressing plasmid to the kidney using ultrasound microbubble technique. Sheep anti-mouse GBM antibody was administered 4d after shRNA activation; and mice were euthanized 10 days later for analysis.
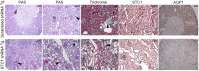
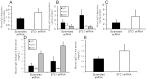
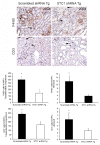
Results: Serum creatinine, proteinuria, albuminuria and urine output were similar 10 days after anti-GBM delivery in both groups; however, anti-GBM antibody delivery to mice with kidney-specific knockdown of STC1 produced severe nephrotoxic nephritis, characterized by severe tubular necrosis, glomerular hyalinosis/necrosis and massive cast formation, while control mice manifested mild tubular injury and crescentic glomerulonephritis. Surprisingly, the expression of cytokines/chemokines and infiltration with T-cells and macrophages were also diminished in STC1 knockdown kidneys. Staining for sheep anti-mouse GBM antibody, deposition of mouse C3 and IgG in the kidney, and antibody response to sheep IgG were equal.
Conclusions: nephrotoxic nephritis after kidney-specific knockdown of STC1 is characterized by severe tubular and glomerular necrosis, possibly due to loss of STC1-mediated pro-survival factors, and we attribute the paucity of inflammation to diminished release of cytokines/chemokines/growth factors from the necrotic epithelium.
Conflict of interest statement
Figures




References
-
- De Niu P, Radman DP, Jaworski EM, Deol H, Gentz R, Su J, et al. (2000) Development of a human stanniocalcin radioimmunoassay: serum and tissue hormone levels and pharmacokinetics in the rat. Mol Cell Endocrinol 162: 131–144. - PubMed
-
- Luo CW, Kawamura K, Klein C, Hsueh AJ (2004) Paracrine regulation of ovarian granulosa cell differentiation by stanniocalcin (STC) 1: mediation through specific STC1 receptors. Mol Endocrinol 18: 2085–2096. - PubMed
-
- McCudden CR, James KA, Hasilo C, Wagner GF (2002) Characterization of mammalian stanniocalcin receptors. Mitochondrial targeting of ligand and receptor for regulation of cellular metabolism. J Biol Chem 277: 45249–45258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

