Diatom-Specific Oligosaccharide and Polysaccharide Structures Help to Unravel Biosynthetic Capabilities in Diatoms
- PMID: 26393622
- PMCID: PMC4584364
- DOI: 10.3390/md13095993
Diatom-Specific Oligosaccharide and Polysaccharide Structures Help to Unravel Biosynthetic Capabilities in Diatoms
Abstract
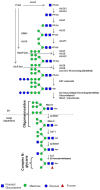
Diatoms are marine organisms that represent one of the most important sources of biomass in the ocean, accounting for about 40% of marine primary production, and in the biosphere, contributing up to 20% of global CO₂ fixation. There has been a recent surge in developing the use of diatoms as a source of bioactive compounds in the food and cosmetic industries. In addition, the potential of diatoms such as Phaeodactylum tricornutum as cell factories for the production of biopharmaceuticals is currently under evaluation. These biotechnological applications require a comprehensive understanding of the sugar biosynthesis pathways that operate in diatoms. Here, we review diatom glycan and polysaccharide structures, thus revealing their sugar biosynthesis capabilities.
Keywords: EPS; diatom; exopolysaccharides; glycan; microalgae; nucleotide sugars; polysaccharide.
Figures






References
-
- Norton T.A., Melkonian M., Andersen R.A. Algal biodiversity. Phycologia. 1996;35:308–326. doi: 10.2216/i0031-8884-35-4-308.1. - DOI
-
- Goldman J.C. Potential role of large oceanic diatoms in new primary production. Deep Sea Res. Part Oceanogr. Res. Pap. 1993;40:159–168. doi: 10.1016/0967-0637(93)90059-C. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

