Tegument Assembly and Secondary Envelopment of Alphaherpesviruses
- PMID: 26393641
- PMCID: PMC4584305
- DOI: 10.3390/v7092861
Tegument Assembly and Secondary Envelopment of Alphaherpesviruses
Abstract
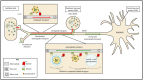
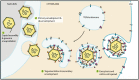
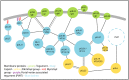
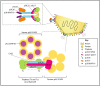
Alphaherpesviruses like herpes simplex virus are large DNA viruses characterized by their ability to establish lifelong latent infection in neurons. As for all herpesviruses, alphaherpesvirus virions contain a protein-rich layer called "tegument" that links the DNA-containing capsid to the glycoprotein-studded membrane envelope. Tegument proteins mediate a diverse range of functions during the virus lifecycle, including modulation of the host-cell environment immediately after entry, transport of virus capsids to the nucleus during infection, and wrapping of cytoplasmic capsids with membranes (secondary envelopment) during virion assembly. Eleven tegument proteins that are conserved across alphaherpesviruses have been implicated in the formation of the tegument layer or in secondary envelopment. Tegument is assembled via a dense network of interactions between tegument proteins, with the redundancy of these interactions making it challenging to determine the precise function of any specific tegument protein. However, recent studies have made great headway in defining the interactions between tegument proteins, conserved across alphaherpesviruses, which facilitate tegument assembly and secondary envelopment. We summarize these recent advances and review what remains to be learned about the molecular interactions required to assemble mature alphaherpesvirus virions following the release of capsids from infected cell nuclei.
Keywords: HSV-1; PrV; herpes simplex virus; pseudorabies virus; virus egress; virus maturation.
Figures
References
-
- Mocarski E.S., Jr. Comparative analysis of herpesvirus-common proteins. In: Arvin A., Campadelli-Fiume G., Mocarski E., Moore P.S., Roizman B., Whitley R., Yamanishi K., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press; Cambridge, UK: 2007. pp. 44–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

