Inflammatory Stress on Autophagy in Peripheral Blood Mononuclear Cells from Patients with Alzheimer's Disease during 24 Months of Follow-Up
- PMID: 26393801
- PMCID: PMC4578953
- DOI: 10.1371/journal.pone.0138326
Inflammatory Stress on Autophagy in Peripheral Blood Mononuclear Cells from Patients with Alzheimer's Disease during 24 Months of Follow-Up
Erratum in
-
Correction: Inflammatory Stress on Autophagy in Peripheral Blood Mononuclear Cells from Patients with Alzheimer's Disease during 24 Months of Follow-Up.PLoS One. 2016 Mar 15;11(3):e0143933. doi: 10.1371/journal.pone.0143933. eCollection 2016. PLoS One. 2016. PMID: 26977934 Free PMC article. No abstract available.
Abstract
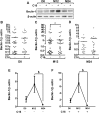
Recent findings indicate that microglia in Alzheimer's disease (AD) is senescent whereas peripheral blood mononuclear cells (PBMCs) could infiltrate the brain to phagocyte amyloid deposits. However, the molecular mechanisms involved in the amyloid peptide clearance remain unknown. Autophagy is a physiological degradation of proteins and organelles and can be controlled by pro-inflammatory cytokines. The purpose of this study was to evaluate the impact of inflammation on autophagy in PBMCs from AD patients at baseline, 12 and 24 months of follow-up. Furthermore, PBMCs from healthy patients were also included and treated with 20 μM amyloid peptide 1-42 to mimic AD environment. For each patient, PBMCs were stimulated with the mitogenic factor, phytohaemagglutin (PHA), and treated with either 1 μM C16 as an anti-inflammatory drug or its vehicle. Autophagic markers (Beclin-1, p62/sequestosome 1 and microtubule-associated protein-light chain 3: LC3) were quantified by western blot and cytokines (Interleukin (IL)-1β, Tumor necrosis Factor (TNF)-α and IL-6) by Luminex X-MAP® technology. Beclin-1 and TNF-α levels were inversely correlated in AD PBMCs at 12 months post-inclusion. In addition, Beclin-1 and p62 increased in the low inflammatory environment induced by C16. Only LC3-I levels were inversely correlated with cognitive decline at baseline. For the first time, this study describes longitudinal changes in autophagic markers in PBMCs of AD patients under an inflammatory environment. Inflammation would induce autophagy in the PBMCs of AD patients while an anti-inflammatory environment could inhibit their autophagic response. However, this positive response could be altered in a highly aggressive environment.
Conflict of interest statement
Figures


References
-
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–94. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

