Antibody-Drug Conjugates (ADCs) Derived from Interchain Cysteine Cross-Linking Demonstrate Improved Homogeneity and Other Pharmacological Properties over Conventional Heterogeneous ADCs
- PMID: 26393951
- PMCID: PMC7755308
- DOI: 10.1021/acs.molpharmaceut.5b00432
Antibody-Drug Conjugates (ADCs) Derived from Interchain Cysteine Cross-Linking Demonstrate Improved Homogeneity and Other Pharmacological Properties over Conventional Heterogeneous ADCs
Abstract
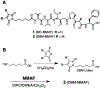
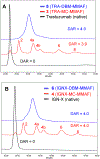
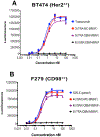
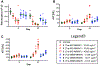
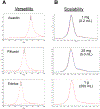
Conventional antibody-drug conjugates (ADCs) are heterogeneous mixtures of chemically distinct molecules that vary in both drugs/antibody (DAR) and conjugation sites. Suboptimal properties of heterogeneous ADCs have led to new site-specific conjugation methods for improving ADC homogeneity. Most site-specific methods require extensive antibody engineering to identify optimal conjugation sites and introduce unique functional groups for conjugation with appropriately modified linkers. Alternative nonrecombinant methods have emerged in which bifunctional linkers are utilized to cross-link antibody interchain cysteines and afford ADCs containing four drugs/antibody. Although these methods have been shown to improve ADC homogeneity and stability in vitro, their effect on the pharmacological properties of ADCs in vivo is unknown. In order to determine the relative impact of interchain cysteine cross-linking on the therapeutic window and other properties of ADCs in vivo, we synthesized a derivative of the known ADC payload, MC-MMAF, that contains a bifunctional dibromomaleimide (DBM) linker instead of a conventional maleimide (MC) linker. The DBM-MMAF derivative was conjugated to trastuzumab and a novel anti-CD98 antibody to afford ADCs containing predominantly four drugs/antibody. The pharmacological properties of the resulting cross-linked ADCs were compared with analogous heterogeneous ADCs derived from conventional linkers. The results demonstrate that DBM linkers can be applied directly to native antibodies, without antibody engineering, to yield highly homogeneous ADCs via cysteine cross-linking. The resulting ADCs demonstrate improved pharmacokinetics, superior efficacy, and reduced toxicity in vivo compared to analogous conventional heterogeneous ADCs.
Keywords: ADC; CD98; DAR; Her2; MMAF; antibody−drug conjugate; auristatin; bifunctional; conjugation; cysteine; dibromomaleimide; disulfide; hinge; homogeneous; interchain; linker; maleimide; site-specific; trastuzumab.
Figures





References
-
- Sievers EL; Senter PD Antibody-drug conjugates in cancer therapy. Annu. Rev. Med 2013, 64, 15–29. - PubMed
-
- Hamblett KJ; Senter PD; Chace DF; Sun MMC; Lenox J; Cerveny CG; Kissler KM; Bernhardt SX; Kopcha AK; Zabinski RF; Meyer DL; Francisco JA Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin. Cancer Res 2004, 10 (20), 7063–70. - PubMed
-
- Boswell CA; Mundo EE; Zhang C; Bumbaca D; Valle NR; Kozak KR; Fourie A; Chuh J; Koppada N; Saad O; Gill H; Shen B-Q; Rubinfeld B; Tibbitts J; Kaur S; Theil F-P; Fielder PJ; Khawli LA; Lin K Impact of Drug Conjugation on Pharmacokinetics and Tissue Distribution of Anti-STEAP1 Antibody-Drug Conjugates in Rats. Bioconjugate Chem. 2011, 22 (10), 1994–2004. - PubMed
-
- Wakankar AA; Feeney MB; Rivera J; Chen Y; Kim M; Sharma VK; Wang YJ Physicochemical Stability of the Antibody-Drug Conjugate Trastuzumab-DM1: Changes due to Modification and Conjugation Processes. Bioconjugate Chem. 2010, 21 (9), 1588–1595. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

