NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid has enhanced chemo-preventive properties compared to aspirin, is gastrointestinal safe with all the classic therapeutic indications
- PMID: 26394025
- PMCID: PMC4656078
- DOI: 10.1016/j.bcp.2015.09.014
NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid has enhanced chemo-preventive properties compared to aspirin, is gastrointestinal safe with all the classic therapeutic indications
Abstract
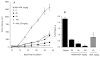
Aspirin is chemopreventive; however, side effects preclude its long-term use. NOSH-aspirin (NBS-1120), a novel hybrid that releases nitric oxide and hydrogen sulfide, was designed to be a safer alternative. Here we compare the gastrointestinal safety, anti-inflammatory, analgesic, anti-pyretic, anti-platelet, and chemopreventive properties of aspirin and NBS-1120 administered orally to rats at equimolar doses. Gastrointestinal safety: 6h post-administration, the number and size of hemorrhagic lesions in stomachs were counted; tissue samples were frozen for PGE2, SOD, and MDA determination. Anti-inflammatory: 1h after drug administration, the volume of carrageenan-induced rat paw edemas was measured for 5h. Anti-pyretic: fever was induced by LPS (ip) an hour before administration of the test drugs, core body temperature was measured hourly for 5h. Analgesic: time-dependent analgesic effects were evaluated by carrageenan-induced hyperalgesia. Antiplatelet: anti-aggregatory effects were studied on collagen-induced platelet aggregation of human platelet-rich plasma. Chemoprevention: nude mice were gavaged daily for 25 days with vehicle, aspirin or NBS-1120. After one week, each mouse was inoculated subcutaneously in the right flank with HT-29 human colon cancer cells. Both agents reduced PGE2 levels in stomach tissue; however, NBS-1120 did not cause any stomach ulcers, whereas aspirin caused significant bleeding. Lipid peroxidation induced by aspirin was higher than that exerted by NBS-1120. SOD activity was significantly inhibited by aspirin but increased by NBS-1120. Both agents showed similar anti-inflammatory, analgesic, anti-pyretic, and anti-platelet activities. Aspirin increased plasma TNFα more than NBS-1120-treated animals. NBS-1120 was better than aspirin as a chemopreventive agent; it dose-dependently inhibited tumor growth and tumor mass.
Keywords: Aspirin; Chemopreventive; GI-sparing; Hydrogen sulfide; Nitric oxide.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors have nothing to disclose except for KK, who has an equity position in Avicenna Pharmaceuticals, Inc. the supplier of NOSH-aspirin used in these studies and to which NBS-1120 is licensed.
Figures


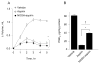

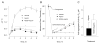

Similar articles
-
NOSH-sulindac (AVT-18A) is a novel nitric oxide- and hydrogen sulfide-releasing hybrid that is gastrointestinal safe and has potent anti-inflammatory, analgesic, antipyretic, anti-platelet, and anti-cancer properties.Redox Biol. 2015 Dec;6:287-296. doi: 10.1016/j.redox.2015.08.012. Epub 2015 Aug 14. Redox Biol. 2015. PMID: 26298203 Free PMC article.
-
NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid is a potent inhibitor of colon cancer cell growth in vitro and in a xenograft mouse model.Biochem Biophys Res Commun. 2012 Mar 16;419(3):523-8. doi: 10.1016/j.bbrc.2012.02.051. Epub 2012 Feb 16. Biochem Biophys Res Commun. 2012. PMID: 22366248
-
Synthesis and anti-cancer potential of the positional isomers of NOSH-aspirin (NBS-1120) a dual nitric oxide and hydrogen sulfide releasing hybrid.Bioorg Med Chem Lett. 2015 Oct 15;25(20):4677-82. doi: 10.1016/j.bmcl.2015.08.023. Epub 2015 Aug 14. Bioorg Med Chem Lett. 2015. PMID: 26323873 Free PMC article.
-
NSAIDs, coxibs, CINOD and H2S-releasing NSAIDs: what lies beyond the horizon.Dig Liver Dis. 2007 Dec;39(12):1043-51. doi: 10.1016/j.dld.2007.09.001. Epub 2007 Nov 7. Dig Liver Dis. 2007. PMID: 17997373 Review.
-
Nitric oxide, aspirin-triggered lipoxins and NO-aspirin in gastric protection.Inflamm Allergy Drug Targets. 2006 Apr;5(2):133-7. doi: 10.2174/187152806776383116. Inflamm Allergy Drug Targets. 2006. PMID: 16613572 Review.
Cited by
-
Hydrogen sulfide-induced post-translational modification as a potential drug target.Genes Dis. 2022 Apr 20;10(5):1870-1882. doi: 10.1016/j.gendis.2022.03.022. eCollection 2023 Sep. Genes Dis. 2022. PMID: 37492730 Free PMC article. Review.
-
The evolving landscape for cellular nitric oxide and hydrogen sulfide delivery systems: A new era of customized medications.Biochem Pharmacol. 2020 Jun;176:113931. doi: 10.1016/j.bcp.2020.113931. Epub 2020 Mar 26. Biochem Pharmacol. 2020. PMID: 32224139 Free PMC article. Review.
-
Development of hydrogen sulfide donors for anti-atherosclerosis therapeutics research: Challenges and future priorities.Front Cardiovasc Med. 2022 Aug 12;9:909178. doi: 10.3389/fcvm.2022.909178. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36035922 Free PMC article. Review.
-
The dichotomous role of H2S in cancer cell biology? Déjà vu all over again.Biochem Pharmacol. 2018 Mar;149:205-223. doi: 10.1016/j.bcp.2018.01.042. Epub 2018 Feb 14. Biochem Pharmacol. 2018. PMID: 29397935 Free PMC article. Review.
-
NOSH-aspirin (NBS-1120) attenuates motor defects and dopaminergic neuron degeneration in a rat model of Parkinson's disease.Eur J Pharmacol. 2025 Sep 5;1002:177733. doi: 10.1016/j.ejphar.2025.177733. Epub 2025 May 28. Eur J Pharmacol. 2025. PMID: 40447068
References
-
- Kune G, Kune S, Watson L. Colorectal cancer risk, chronic illnesses, operations, and medications: case-control results from the Melbourne Colorectal Cancer Study. Cancer Research. 1988;48:4399–404. - PubMed
-
- Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–9. - PubMed
-
- Benamouzig R, Deyra J, Martin A, Girard B, Jullian E, Piednoir B, et al. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology. 2003;125:328–36. - PubMed
-
- Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–90. - PubMed
-
- Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

