Significant Effects of Oral Phenylbutyrate and Vitamin D3 Adjunctive Therapy in Pulmonary Tuberculosis: A Randomized Controlled Trial
- PMID: 26394045
- PMCID: PMC4578887
- DOI: 10.1371/journal.pone.0138340
Significant Effects of Oral Phenylbutyrate and Vitamin D3 Adjunctive Therapy in Pulmonary Tuberculosis: A Randomized Controlled Trial
Abstract
Background: Development of new tuberculosis (TB) drugs and alternative treatment strategies are urgently required to control the global spread of TB. Previous results have shown that vitamin D3 (vitD3) and 4-phenyl butyrate (PBA) are potent inducers of the host defense peptide LL-37 that possess anti-mycobacterial effects.
Objective: To examine if oral adjunctive therapy with 5,000IU vitD3 or 2x500 mg PBA or PBA+vitD3 to standard chemotherapy would lead to enhanced recovery in sputum smear-positive pulmonary TB patients.
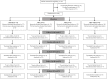
Methods: Adult TB patients (n = 288) were enrolled in a randomized, double-blind, placebo-controlled trial conducted in Bangladesh. Primary endpoints included proportions of patients with a negative sputum culture at week 4 and reduction in clinical symptoms at week 8. Clinical assessments and sputum smear microscopy were performed weekly up to week 4, fortnightly up to week 12 and at week 24; TB culture was performed at week 0, 4 and 8; concentrations of LL-37 in cells, 25-hydroxyvitamin D3 (25(OH)D3) in plasma and ex vivo bactericidal function of monocyte-derived macrophages (MDM) were determined at week 0, 4, 8, 12 and additionally at week 24 for plasma 25(OH)D3.
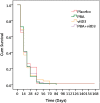
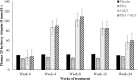
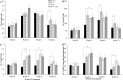
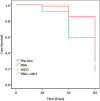
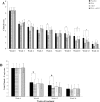
Results: At week 4, 71% (46/65) of the patients in the PBA+vitD3-group (p = 0.001) and 61.3% (38/62) in the vitD3-group (p = 0.032) were culture negative compared to 42.2% (27/64) in the placebo-group. The odds of sputum culture being negative at week 4 was 3.42 times higher in the PBA+vitD3-group (p = 0.001) and 2.2 times higher in vitD3-group (p = 0.032) compared to placebo. The concentration of LL-37 in MDM was significantly higher in the PBA-group compared to placebo at week 12 (p = 0.034). Decline in intracellular Mtb growth in MDM was earlier in the PBA-group compared to placebo (log rank 11.38, p = 0.01).
Conclusion: Adjunct therapy with PBA+vitD3 or vitD3 or PBA to standard short-course therapy demonstrated beneficial effects towards clinical recovery and holds potential for host-directed-therapy in the treatment of TB.
Trial registration: clinicaltrials.gov NCT01580007.
Conflict of interest statement
Figures

References
-
- Organization WH. Global Tuberculosis Report 2014. France: World Health Organization, 2014 WHO/HTM/TB/2014.08.
-
- Lönnroth K. The looming co-epidemic of TB-diabetes: A call to action. International Union Against Tuberculosis and Lung Disease, 2014.
-
- Organization WH. Drug-resistant TB—surveillance & response France: World Health Organization, 2014. Contract No.: WHO/HQ/TB/2014.12.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

