BamA Alone Accelerates Outer Membrane Protein Folding In Vitro through a Catalytic Mechanism
- PMID: 26394056
- PMCID: PMC4613867
- DOI: 10.1021/acs.biochem.5b00950
BamA Alone Accelerates Outer Membrane Protein Folding In Vitro through a Catalytic Mechanism
Abstract
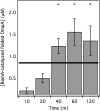
β-Barrel assembly machinery protein A (BamA) plays a critical role in the biogenesis of outer membrane proteins (OMPs); however, a mechanistic understanding of its function is lacking. Here, we report an in vitro assay that investigates whether the mechanism of BamA-catalyzed OMP folding is stoichiometric or catalytic. We found that BamA accelerates the folding of OMPs in vitro via a catalytic mechanism, similar to the activity of the full multiprotein β-barrel assembly machinery (BAM) complex in vivo. As BamA alone can repeatedly facilitate the folding of OMPs, we suggest the additional BAM components accelerate this basal activity to biologically relevant time scales.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

