Molecular magnetic resonance imaging in cancer
- PMID: 26394751
- PMCID: PMC4579835
- DOI: 10.1186/s12967-015-0659-x
Molecular magnetic resonance imaging in cancer
Abstract
The ability to identify key biomolecules and molecular changes associated with cancer malignancy and the capacity to monitor the therapeutic outcome against these targets is critically important for cancer treatment. Recent developments in molecular imaging based on magnetic resonance (MR) techniques have provided researchers and clinicians with new tools to improve most facets of cancer care. Molecular imaging is broadly described as imaging techniques used to detect molecular signature at the cellular and gene expression levels. This article reviews both established and emerging molecular MR techniques in oncology and discusses the potential of these techniques in improving the clinical cancer care. It also discusses how molecular MR, in conjunction with other structural and functional MR imaging techniques, paves the way for developing tailored treatment strategies to enhance cancer care.
Figures
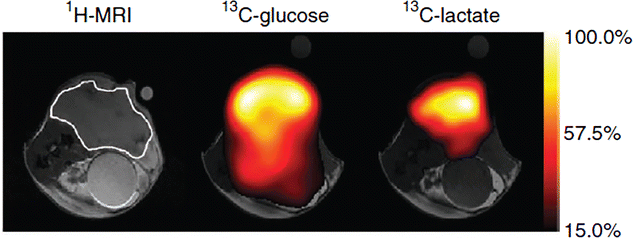
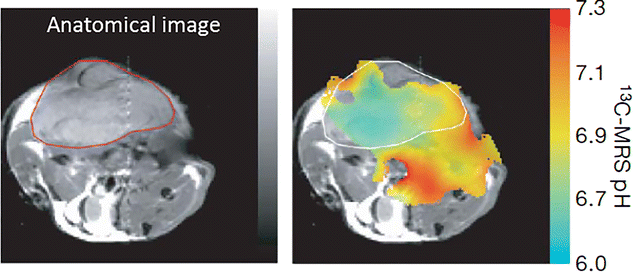
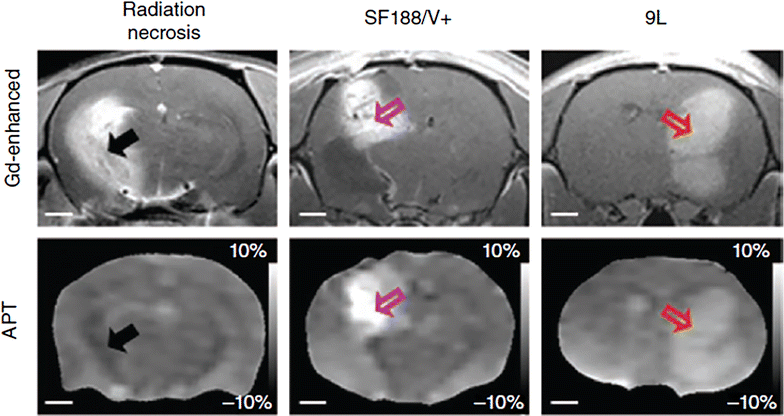
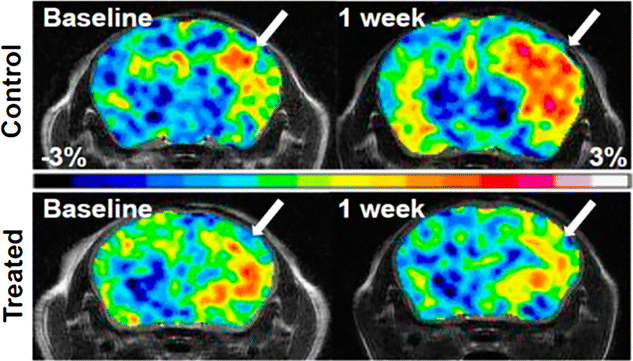
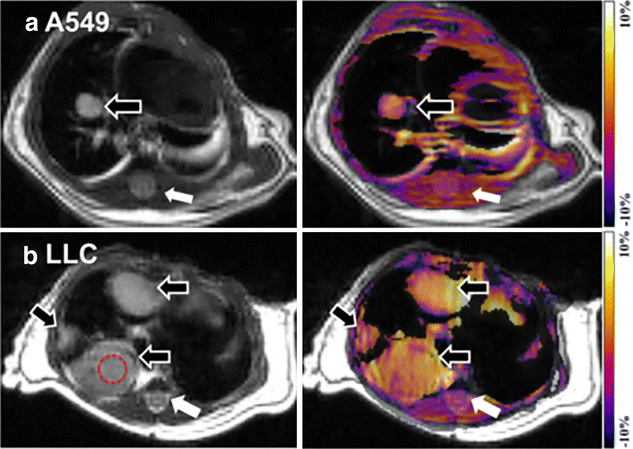
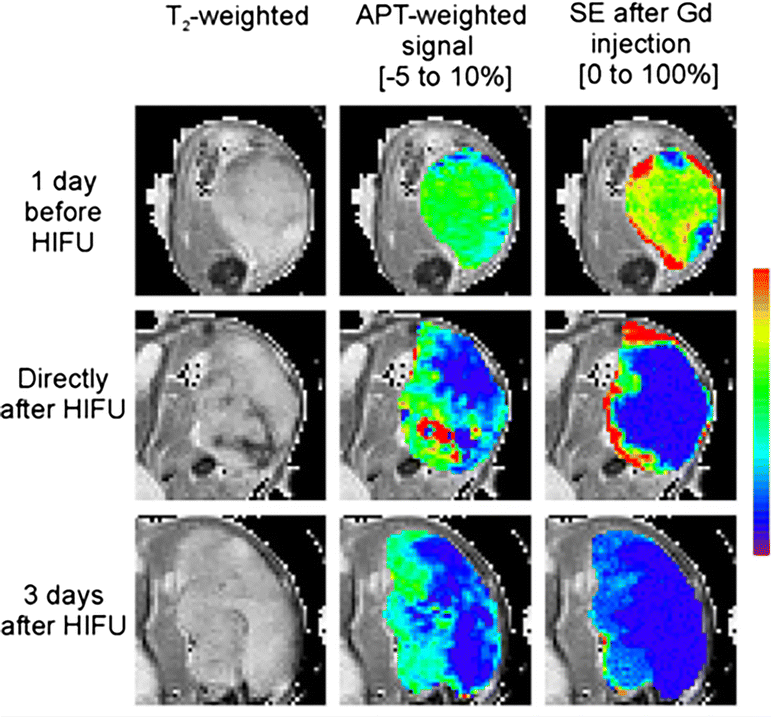
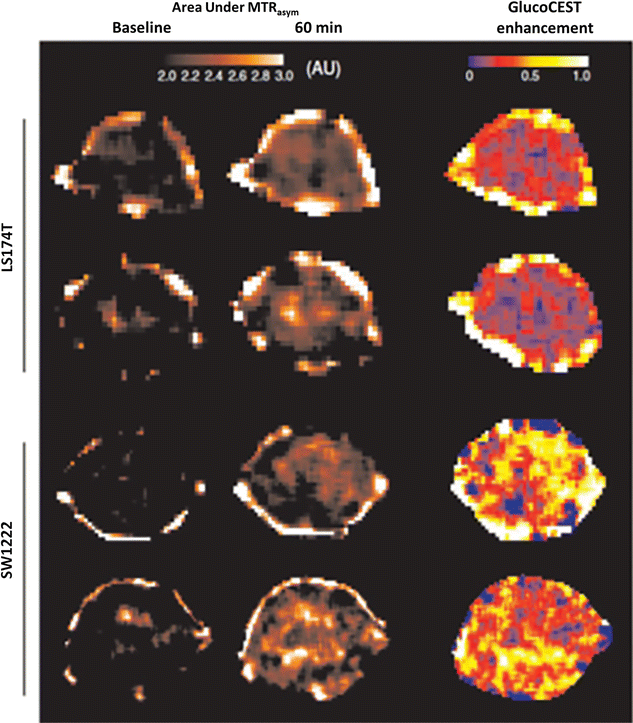

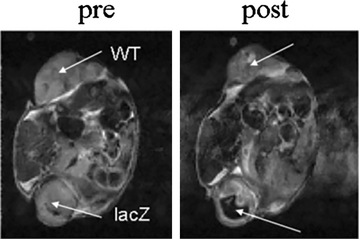
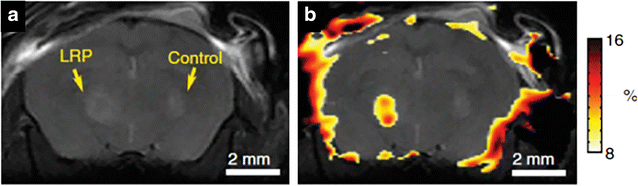
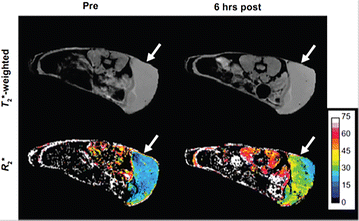
References
-
- Vangestel C, Van de Wiele C, Mees G, Mertens K, Staelens S, Reutelingsperger C, Pauwels P, Van Damme N, Peeters M. Single-photon emission computed tomographic imaging of the early time course of therapy-induced cell death using technetium 99 m tricarbonyl His-annexin A5 in a colorectal cancer xenograft model. Mol Imaging. 2012;11:135–147. - PubMed
-
- Weber WA. Use of PET for monitoring cancer therapy and for predicting outcome. J Nucl Med. 2005;46:983–995. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

