Revising the embryonic origin of thyroid C cells in mice and humans
- PMID: 26395490
- PMCID: PMC4631767
- DOI: 10.1242/dev.126581
Revising the embryonic origin of thyroid C cells in mice and humans
Abstract
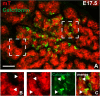
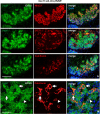
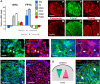
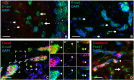
Current understanding infers a neural crest origin of thyroid C cells, the major source of calcitonin in mammals and ancestors to neuroendocrine thyroid tumors. The concept is primarily based on investigations in quail-chick chimeras involving fate mapping of neural crest cells to the ultimobranchial glands that regulate Ca(2+) homeostasis in birds, reptiles, amphibians and fishes, but whether mammalian C cell development involves a homologous ontogenetic trajectory has not been experimentally verified. With lineage tracing, we now provide direct evidence that Sox17+ anterior endoderm is the only source of differentiated C cells and their progenitors in mice. Like many gut endoderm derivatives, embryonic C cells were found to coexpress pioneer factors forkhead box (Fox) a1 and Foxa2 before neuroendocrine differentiation takes place. In the ultimobranchial body epithelium emerging from pharyngeal pouch endoderm in early organogenesis, differential Foxa1/Foxa2 expression distinguished two spatially separated pools of C cell precursors with different growth properties. A similar expression pattern was recapitulated in medullary thyroid carcinoma cells in vivo, consistent with a growth-promoting role of Foxa1. In contrast to embryonic precursor cells, C cell-derived tumor cells invading the stromal compartment downregulated Foxa2, foregoing epithelial-to-mesenchymal transition designated by loss of E-cadherin; both Foxa2 and E-cadherin were re-expressed at metastatic sites. These findings revise mammalian C cell ontogeny, expand the neuroendocrine repertoire of endoderm and redefine the boundaries of neural crest diversification. The data further underpin distinct functions of Foxa1 and Foxa2 in both embryonic and tumor development.
Keywords: Endoderm; Foxa1; Foxa2; Medullary thyroid cancer; Neural crest; Neuroendocrine.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

