Metabolism of an Alkylated Polycyclic Aromatic Hydrocarbon 5-Methylchrysene in Human Hepatoma (HepG2) Cells
- PMID: 26395544
- PMCID: PMC4709222
- DOI: 10.1021/acs.chemrestox.5b00256
Metabolism of an Alkylated Polycyclic Aromatic Hydrocarbon 5-Methylchrysene in Human Hepatoma (HepG2) Cells
Abstract
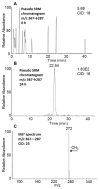
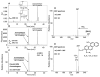
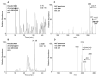
Exposure to polycyclic aromatic hydrocarbons (PAHs) in the food chain is the major human health hazard associated with the Deepwater Horizon oil spill. C1-chrysenes are representative PAHs present in the crude oil and have been detected in contaminated sea food in amounts that exceed their permissible safety thresholds. We describe the metabolism of the most carcinogenic C1-chrysene regioisomer, 5-methylchrysene (5-MC), in human HepG2 cells. The structures of the metabolites were identified by HPLC-UV-fluorescence detection and LC-MS/MS. 5-MC-tetraol, a signature metabolite of the diol-epoxide pathway, was identified as reported previously. Novel O-monosulfonated-5-MC-catechol isomers and O-monomethyl-O-monosulfonated-5-MC-catechol were discovered, and evidence for their precursor ortho-quinones was obtained. The identities of O-monosulfonated-5-MC-1,2-catechol, O-monomethyl-O-monosulfonated-5-MC-1,2-catechol, and 5-MC-1,2-dione were validated by comparison to authentic synthesized standards. Dual metabolic activation of 5-MC involving the formation of bis-electrophiles, i.e., a mono-diol-epoxide and a mono-ortho-quinone within the same structure, bis-diol-epoxides, and bis-ortho-quinones is reported for the first time. Evidence was also obtained for minor metabolic conversion of 5-MC to form monohydroxylated-quinones and bis-phenols. The identification of 5-MC-tetraol, O-monosulfonated-5-MC-1,2-catechol, O-monomethyl-O-monosulfonated-5-MC-1,2-catechol, and 5-MC-1,2-dione supports metabolic activation of 5-MC by P450 and AKR isozymes followed by metabolic detoxification of the ortho-quinone through interception of redox cycling by COMT and SULT isozymes. The major metabolites, O-monosulfonated-catechols and tetraols, could be used as biomarkers of human exposure to 5-MC resulting from oil spills.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Joye SB, MacDonald IR, Leifer I, Asper V. Magnitude and oxidation potential of hydrocarbon gases released from the BP oil well blowout. Nat Geosci. 2011;4:160–164.
-
- Goldstein BD, Osofsky HJ, Lichtveld MY. The Gulf oil spill. N Engl J Med. 2011;364:1334–1348. - PubMed
-
- National Institute of Standards and Technology Certificate of Analysis: Standard Reference Material (SRM) 2779, Gulf of Mexico Crude Oil. National Institue of Standards and Technology; Gaithersburg, MD: 2012. pp. 1–7.
-
- Ylitalo GM, Krahn MM, Dickhoff WW, Stein JE, Walker CC, Lassitter CL, Garrett ES, Desfosse LL, Mitchell KM, Noble BT, Wilson S, Beck NB, Benner RA, Koufopoulos PN, Dickey RW. Federal seafood safety response to the Deepwater Horizon oil spill. Proc Natl Acad Sci U S A. 2012;109:20274–20279. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

