Rodent models for resolving extremes of exercise and health
- PMID: 26395598
- PMCID: PMC4729696
- DOI: 10.1152/physiolgenomics.00077.2015
Rodent models for resolving extremes of exercise and health
Abstract
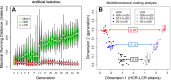
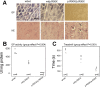
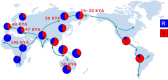
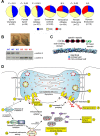
The extremes of exercise capacity and health are considered a complex interplay between genes and the environment. In general, the study of animal models has proven critical for deep mechanistic exploration that provides guidance for focused and hypothesis-driven discovery in humans. Hypotheses underlying molecular mechanisms of disease and gene/tissue function can be tested in rodents to generate sufficient evidence to resolve and progress our understanding of human biology. Here we provide examples of three alternative uses of rodent models that have been applied successfully to advance knowledge that bridges our understanding of the connection between exercise capacity and health status. First we review the strong association between exercise capacity and all-cause morbidity and mortality in humans through artificial selection on low and high exercise performance in the rat and the consequent generation of the "energy transfer hypothesis." Second we review specific transgenic and knockout mouse models that replicate the human disease condition and performance. This includes human glycogen storage diseases (McArdle and Pompe) and α-actinin-3 deficiency. Together these rodent models provide an overview of the advancements of molecular knowledge required for clinical translation. Continued study of these models in conjunction with human association studies will be critical to resolving the complex gene-environment interplay linking exercise capacity, health, and disease.
Keywords: disease; exercise; genetic loci; humans; mice; performance; rats.
Copyright © 2016 the American Physiological Society.
Figures

References
-
- Alfred T, Ben-Shlomo Y, Cooper R, Hardy R, Cooper C, Deary IJ, Gunnell D, Harris SE, Kumari M, Martin RM, Moran CN, Pitsiladis YP, Ring SM, Sayer AA, Smith GD, Starr JM, Kuh D, Day IN. ACTN3 genotype, athletic status, and life course physical capability: meta-analysis of the published literature and findings from nine studies. Hum Mutat 32: 1008–1018, 2011. - PMC - PubMed
-
- Andersen ST, Duno M, Schwartz M, Vissing J. Do carriers of PYGM mutations have symptoms of McArdle disease? Neurology 67: 716–718, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

