CD8+ T cells undergo activation and programmed death-1 repression in the liver of aged Ae2a,b-/- mice favoring autoimmune cholangitis
- PMID: 26396175
- PMCID: PMC4745679
- DOI: 10.18632/oncotarget.5665
CD8+ T cells undergo activation and programmed death-1 repression in the liver of aged Ae2a,b-/- mice favoring autoimmune cholangitis
Abstract
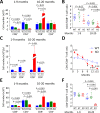
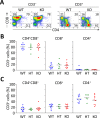
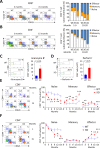
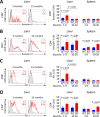
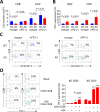
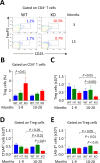
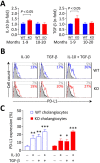
Primary biliary cirrhosis (PBC) is a chronic cholestatic disease of unknown etiopathogenesis showing progressive autoimmune-mediated cholangitis. In PBC patients, the liver and lymphocytes exhibit diminished expression of AE2/SLC4A2, a Cl-/HCO3- anion exchanger involved in biliary bicarbonate secretion and intracellular pH regulation. Decreased AE2 expression may be pathogenic as Ae2a,b(-/-) mice reproduce hepatobiliary and immunological features resembling PBC. To understand the role of AE2 deficiency for autoimmunity predisposition we focused on the phenotypic changes of T cells that occur over the life-span of Ae2a,b(-/-) mice. At early ages (1-9 months), knockout mice had reduced numbers of intrahepatic T cells, which exhibited increased activation, programmed-cell-death (PD)-1 expression, and apoptosis. Moreover, young knockouts had upregulated PD-1 ligand (PD-L1) on bile-duct cells, and administration of neutralizing anti-PD-L1 antibodies prevented their intrahepatic T-cell deletion. Older (≥ 10 months) knockouts, however, showed intrahepatic accumulation of cytotoxic CD8(+) T cells with downregulated PD-1 and diminished apoptosis. In-vitro DNA demethylation with 5-aza-2'-deoxycytidine partially reverted PD-1 downregulation of intrahepatic CD8(+) T cells from aged knockouts.
Conclusion: Early in life, AE2 deficiency results in intrahepatic T-cell activation and PD-1/PD-L1 mediated deletion. With aging, intrahepatic CD8+ T cells epigenetically suppress PD-1, and their consequential expansion and further activation favor autoimmune cholangitis.
Keywords: Immune response; Immunity; Immunology and Microbiology Section; Na+-independent Cl−/HCO3− anion exchanger AE2; age-related changes; intracellular pH homeostasis; mouse model of autoimmune cholangitis; self-tolerance breakdown.
Conflict of interest statement
The authors have declared no financial conflict of interest.
Figures




Similar articles
-
Promoter hypermethylation of the AE2/SLC4A2 gene in PBC.JHEP Rep. 2019 Jun 7;1(3):145-153. doi: 10.1016/j.jhepr.2019.05.006. eCollection 2019 Sep. JHEP Rep. 2019. PMID: 32039364 Free PMC article.
-
Ae2a,b-deficient mice develop antimitochondrial antibodies and other features resembling primary biliary cirrhosis.Gastroenterology. 2008 May;134(5):1482-93. doi: 10.1053/j.gastro.2008.02.020. Epub 2008 Feb 14. Gastroenterology. 2008. PMID: 18471521
-
Anion exchanger 2 is critical for CD8(+) T cells to maintain pHi homeostasis and modulate immune responses.Eur J Immunol. 2014 May;44(5):1341-51. doi: 10.1002/eji.201344218. Epub 2014 Mar 11. Eur J Immunol. 2014. PMID: 24515893
-
Role of the anion exchanger 2 in the pathogenesis and treatment of primary biliary cirrhosis.Dig Dis. 2011;29(1):103-12. doi: 10.1159/000324144. Epub 2011 Jun 17. Dig Dis. 2011. PMID: 21691115 Review.
-
Primary biliary cholangitis: pathogenic mechanisms.Curr Opin Gastroenterol. 2021 Mar 1;37(2):91-98. doi: 10.1097/MOG.0000000000000703. Curr Opin Gastroenterol. 2021. PMID: 33332913 Free PMC article. Review.
Cited by
-
Ion channelopathies of the immune system.Curr Opin Immunol. 2018 Jun;52:39-50. doi: 10.1016/j.coi.2018.03.021. Epub 2018 Apr 7. Curr Opin Immunol. 2018. PMID: 29635109 Free PMC article. Review.
-
Mouse models of SLC4-linked disorders of HCO3--transporter dysfunction.Am J Physiol Cell Physiol. 2018 May 1;314(5):C569-C588. doi: 10.1152/ajpcell.00301.2017. Epub 2018 Jan 31. Am J Physiol Cell Physiol. 2018. PMID: 29384695 Free PMC article. Review.
-
Animal models of primary biliary cholangitis: status and challenges.Cell Biosci. 2023 Nov 22;13(1):214. doi: 10.1186/s13578-023-01170-9. Cell Biosci. 2023. PMID: 37993960 Free PMC article. Review.
-
Structural and functional insights into the lipid regulation of human anion exchanger 2.Nat Commun. 2024 Jan 26;15(1):759. doi: 10.1038/s41467-024-44966-0. Nat Commun. 2024. PMID: 38272905 Free PMC article.
-
The Influence of Heterochronic Non-Myeloablative Bone Marrow Transplantation on the Immune System, Frailty, General Health, and Longevity of Aged Murine Recipients.Biomolecules. 2022 Apr 18;12(4):595. doi: 10.3390/biom12040595. Biomolecules. 2022. PMID: 35454183 Free PMC article.
References
-
- Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. - PubMed
-
- Poupon R. Primary biliary cirrhosis: a 2010 update. J Hepatol. 2010;52:745–758. - PubMed
-
- Tsuda M, Ambrosini YM, Zhang W, Yang GX, Ando Y, Rong G, Tsuneyama K, Sumida K, Shimoda S, Bowlus CL, Leung PS, He XS, Coppel RL, et al. Fine phenotypic and functional characterization of effector cluster of differentiation 8 positive T cells in human patients with primary biliary cirrhosis. Hepatology. 2011;54:1293–1302. - PMC - PubMed
-
- He XS, Ansari AA, Ridgway WM, Coppel RL, Gershwin ME. New insights to the immunopathology and autoimmune responses in primary biliary cirrhosis. Cell Immunol. 2006;239:1–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

