Radiation-induced lung damage promotes breast cancer lung-metastasis through CXCR4 signaling
- PMID: 26396176
- PMCID: PMC4694940
- DOI: 10.18632/oncotarget.5666
Radiation-induced lung damage promotes breast cancer lung-metastasis through CXCR4 signaling
Abstract
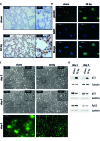
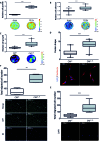
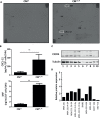
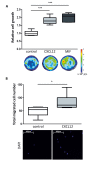
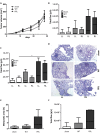
Radiotherapy is a mainstay in the postoperative treatment of breast cancer as it reduces the risks of local recurrence and mortality after both conservative surgery and mastectomy. Despite recent efforts to decrease irradiation volumes through accelerated partial irradiation techniques, late cardiac and pulmonary toxicity still occurs after breast irradiation. The importance of this pulmonary injury towards lung metastasis is unclear. Preirradiation of lung epithelial cells induces DNA damage, p53 activation and a secretome enriched in the chemokines SDF-1/CXCL12 and MIF. Irradiated lung epithelial cells stimulate adhesion, spreading, growth, and (transendothelial) migration of human MDA-MB-231 and murine 4T1 breast cancer cells. These metastasis-associated cellular activities were largely mimicked by recombinant CXCL12 and MIF. Moreover, an allosteric inhibitor of the CXCR4 receptor prevented the metastasis-associated cellular activities stimulated by the secretome of irradiated lung epithelial cells. Furthermore, partial (10%) irradiation of the right lung significantly stimulated breast cancer lung-specific metastasis in the syngeneic, orthotopic 4T1 breast cancer model.Our results warrant further investigation of the potential pro-metastatic effects of radiation and indicate the need to develop efficient drugs that will be successful in combination with radiotherapy to prevent therapy-induced spread of cancer cells.
Keywords: AMD3100; CXCL12; MIF; radiotherapy; triple-negative breast cancer.
Conflict of interest statement
There are no conflicts of interests.
Figures


References
-
- McGale P, Taylor C, Correa C, Cutter D, Duane F, Ewertz M, Gray R, Mannu G, Peto R, Whelan T, Wang Y, Wang Z, Darby S. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. The Lancet. 2014;383:2127–2135. - PMC - PubMed
-
- Moran MS. Radiation therapy in the locoregional treatment of triple-negative breast cancer. The Lancet Oncology. 2015;16:113–122. - PubMed
-
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. The Lancet. 2005;366:2087–2106. - PubMed
-
- Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, et al. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. The Lancet. 2011;378:1707–1716. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

