c-Type Cytochrome Assembly Is a Key Target of Copper Toxicity within the Bacterial Periplasm
- PMID: 26396241
- PMCID: PMC4600104
- DOI: 10.1128/mBio.01007-15
c-Type Cytochrome Assembly Is a Key Target of Copper Toxicity within the Bacterial Periplasm
Abstract
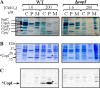
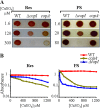
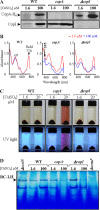
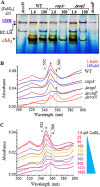
In the absence of a tight control of copper entrance into cells, bacteria have evolved different systems to control copper concentration within the cytoplasm and the periplasm. Central to these systems, the Cu(+) ATPase CopA plays a major role in copper tolerance and translocates copper from the cytoplasm to the periplasm. The fate of copper in the periplasm varies among species. Copper can be sequestered, oxidized, or released outside the cells. Here we describe the identification of CopI, a periplasmic protein present in many proteobacteria, and show its requirement for copper tolerance in Rubrivivax gelatinosus. The ΔcopI mutant is more susceptible to copper than the Cu(+) ATPase copA mutant. CopI is induced by copper, localized in the periplasm and could bind copper. Interestingly, copper affects cytochrome c membrane complexes (cbb3 oxidase and photosystem) in both ΔcopI and copA-null mutants, but the causes are different. In the copA mutant, heme and chlorophyll synthesis are affected, whereas in ΔcopI mutant, the decrease is a consequence of impaired cytochrome c assembly. This impact on c-type cytochromes would contribute also to the copper toxicity in the periplasm of the wild-type cells when they are exposed to high copper concentrations.
Importance: Copper is an essential cation required as a cofactor in enzymes involved in vital processes such as respiration, photosynthesis, free radical scavenging, and pathogenesis. However, copper is highly toxic and has been implicated in disorders in all organisms, including humans, because it can catalyze the production of toxic reactive oxygen species and targets various biosynthesis pathways. Identifying copper targets, provides insights into copper toxicity and homeostatic mechanisms for copper tolerance. In this work, we describe for the first time a direct effect of excess copper on cytochrome c assembly. We show that excess copper specifically affects periplasmic and membrane cytochromes c, thus suggesting that the copper toxicity targets c-type cytochrome biogenesis.
Copyright © 2015 Durand et al.
Figures



References
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
