Active mixing of complex fluids at the microscale
- PMID: 26396254
- PMCID: PMC4603479
- DOI: 10.1073/pnas.1509224112
Active mixing of complex fluids at the microscale
Abstract
Mixing of complex fluids at low Reynolds number is fundamental for a broad range of applications, including materials assembly, microfluidics, and biomedical devices. Of these materials, yield stress fluids (and gels) pose the most significant challenges, especially when they must be mixed in low volumes over short timescales. New scaling relationships between mixer dimensions and operating conditions are derived and experimentally verified to create a framework for designing active microfluidic mixers that can efficiently homogenize a wide range of complex fluids. Active mixing printheads are then designed and implemented for multimaterial 3D printing of viscoelastic inks with programmable control of local composition.
Keywords: 3D printing; graded materials; microfluidic mixing; yield stress fluids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
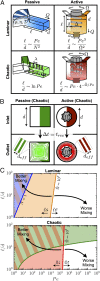
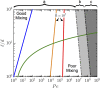



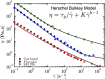
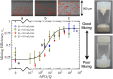



References
-
- Nguyen N-T, Wu Z. Micromixers—a review. J Micromech Microeng. 2005;15(2):R1–R16.
-
- Hessel V, Löwe H, Schönfeld F. Micromixers—a review on passive and active mixing principles. Chem Eng Sci. 2005;60(8):2479–2501.
-
- El-Ali J, Sorger PK, Jensen KF. Cells on chips. Nature. 2006;442(7101):403–411. - PubMed
-
- Haswell SJ, Watts P. Green chemistry: Synthesis in micro reactors. Green Chem. 2003;5:240–249. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

