Control over overall shape and size in de novo designed proteins
- PMID: 26396255
- PMCID: PMC4603489
- DOI: 10.1073/pnas.1509508112
Control over overall shape and size in de novo designed proteins
Abstract
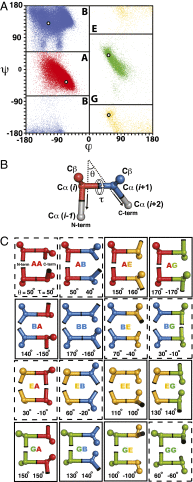
We recently described general principles for designing ideal protein structures stabilized by completely consistent local and nonlocal interactions. The principles relate secondary structure patterns to tertiary packing motifs and enable design of different protein topologies. To achieve fine control over protein shape and size within a particular topology, we have extended the design rules by systematically analyzing the codependencies between the lengths and packing geometry of successive secondary structure elements and the backbone torsion angles of the loop linking them. We demonstrate the control afforded by the resulting extended rule set by designing a series of proteins with the same fold but considerable variation in secondary structure length, loop geometry, β-strand registry, and overall shape. Solution NMR structures of four designed proteins for two different folds show that protein shape and size can be precisely controlled within a given protein fold. These extended design principles provide the foundation for custom design of protein structures performing desired functions.
Keywords: control protein shape; de novo design; ideal protein; protein design.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

