Dichotomous roles for externalized cardiolipin in extracellular signaling: Promotion of phagocytosis and attenuation of innate immunity
- PMID: 26396268
- PMCID: PMC4760701
- DOI: 10.1126/scisignal.aaa6179
Dichotomous roles for externalized cardiolipin in extracellular signaling: Promotion of phagocytosis and attenuation of innate immunity
Abstract
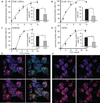
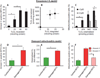
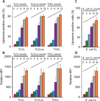
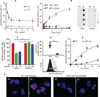
Among the distinct molecular signatures present in the mitochondrion is the tetra-acylated anionic phospholipid cardiolipin, a lipid also present in primordial, single-cell bacterial ancestors of mitochondria and multiple bacterial species today. Cardiolipin is normally localized to the inner mitochondrial membrane; however, when cardiolipin becomes externalized to the surface of dysregulated mitochondria, it promotes inflammasome activation and stimulates the elimination of damaged or nonfunctional mitochondria by mitophagy. Given the immunogenicity of mitochondrial and bacterial membranes that are released during sterile and pathogen-induced trauma, we hypothesized that cardiolipins might function as "eat me" signals for professional phagocytes. In experiments with macrophage cell lines and primary macrophages, we found that membranes with mitochondrial or bacterial cardiolipins on their surface were engulfed through phagocytosis, which depended on the scavenger receptor CD36. Distinct from this process, the copresentation of cardiolipin with the Toll-like receptor 4 (TLR4) agonist lipopolysaccharide dampened TLR4-stimulated production of cytokines. These data suggest that externalized, extracellular cardiolipins play a dual role in host-host and host-pathogen interactions by promoting phagocytosis and attenuating inflammatory immune responses.
Copyright © 2015, American Association for the Advancement of Science.
Figures


References
-
- Diekmann Y, Pereira-Leal JB. Evolution of intracellular compartmentalization. Biochem. J. 2013;449:319–331. - PubMed
-
- Senovilla L, Galluzzi L, Zitvogel L, Kroemer G. Immunosurveillance as a regulator of tissue homeostasis. Trends Immunol. 2013;34:471–481. - PubMed
-
- Thaiss CA, Levy M, Suez J, Elinav E. The interplay between the innate immune system and the microbiota. Curr. Opin. Immunol. 2014;26:41–48. - PubMed
-
- Michlewska S, McColl A, Rossi AG, Megson IL, Dransfield I. Clearance of dying cells and autoimmunity. Autoimmunity. 2007;40:267–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA165065/CA/NCI NIH HHS/United States
- R01 OH008282/OH/NIOSH CDC HHS/United States
- ES020693/ES/NIEHS NIH HHS/United States
- NS076511/NS/NINDS NIH HHS/United States
- CA165065/CA/NCI NIH HHS/United States
- P01 HL114453/HL/NHLBI NIH HHS/United States
- R01 HL096376/HL/NHLBI NIH HHS/United States
- R01 HL081784/HL/NHLBI NIH HHS/United States
- NS061817/NS/NINDS NIH HHS/United States
- I01 BX002200/BX/BLRD VA/United States
- R01 ES020693/ES/NIEHS NIH HHS/United States
- U19 AI068021/AI/NIAID NIH HHS/United States
- HL086884/HL/NHLBI NIH HHS/United States
- P30 CA047904/CA/NCI NIH HHS/United States
- U19AIO68021/PHS HHS/United States
- P30CA047904/CA/NCI NIH HHS/United States
- R37 NS061817/NS/NINDS NIH HHS/United States
- UH2 HL123502/HL/NHLBI NIH HHS/United States
- R01 NS076511/NS/NINDS NIH HHS/United States
- OH008282/OH/NIOSH CDC HHS/United States
- R01 NS061817/NS/NINDS NIH HHS/United States
- R01 HL086884/HL/NHLBI NIH HHS/United States
- R01 HL098174/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

