Protein tyrosine phosphatase SHP-1 sensitizes EGFR/HER-2 positive breast cancer cells to trastuzumab through modulating phosphorylation of EGFR and HER-2
- PMID: 26396531
- PMCID: PMC4576899
- DOI: 10.2147/OTT.S82225
Protein tyrosine phosphatase SHP-1 sensitizes EGFR/HER-2 positive breast cancer cells to trastuzumab through modulating phosphorylation of EGFR and HER-2
Abstract
Background: Trastuzumab resistance in HER-2 positive breast cancer cells is closely related to overexpression of both epidermal growth factor receptor (EGFR) and human epidermal receptor (HER-2). SHP-1 has been demonstrated to downregulate tyrosine kinase activity including EGFR via its phosphatase function, but its effect on HER-2 activity is still unknown. Here, we examined the hypothesis that SHP-1 enhances the anticancer efficacy of trastuzumab in EGFR/HER-2 positive breast cancer cells through combining dual inhibition of EGFR and HER-2.
Methods: Trastuzumab-resistant breast cancer SKBr-3 cells were generated by long-term in vitro culture of SKBr-3cells in the presence of trastuzumab. The SHP-1 was ectopically expressed by stable transfection. The activity and expression of EGFR, HER-2, and downstream signaling pathways were tested by Western blot. Cell viability was examined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, and apoptosis was examined by flow cytometry. The binding between SHP-1 and EGFR/HER-2 was evaluated by immunoprecipitation assay and bimolecular fluorescence complementation. The effects of SHP-1 on tumorigenicity and trastuzumab sensitivity were confirmed via in vivo xenograft model.
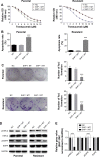
Results: Trastuzumab-resistant SKBr-3 cells showed aberrant co-expression of EGFR and HER-2. Introduction of wild-type SHP-1 inhibited cell proliferation, clone formation, and promoted the apoptosis induced by trastuzumab. Meanwhile, SHP-1 overexpression reduced phosphorylation levels of EGFR and HER-2 both in parental and trastuzumab-resistant SKBr-3 cells. In vivo study showed an increased antitumor effect of trastuzumab in SHP-1 overexpressed xenografts. At last, we discovered that SHP-1 can make complexes with both EGFR and HER-2, and both phospho-EGFR and phosphor-HER-2 levels in wild-type SHP-1 immunoprecipitates were less than those in phosphatase-inactive SHP-1 (C453S) immunoprecipitates, indicating that EGFR and HER-2 are potential substrates of SHP-1.
Conclusion: Taken together, we have demonstrated that the SHP-1 is a negative regulatory factor of the tyrosine kinase activity of HER-2 and EGFR through inhibiting phosphorylation. Dual targeting of EGFR and HER-2, by combining trastuzumab with SHP-1 overexpression, may improve response in HER-2 overexpressing breast cancer cells that also express high levels of EGFR.
Keywords: EGFR; HER-2; SHP-1; breast cancer; drug resistance; trastuzumab.
Figures



References
-
- Nahleh ZA. Hormonal therapy for male breast cancer: a different approach for a different disease. Cancer Treat Rev. 2006;32:101–105. - PubMed
-
- Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2013. Ann Oncol. 2013;24:792–800. - PubMed
-
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. - PubMed
-
- Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. - PubMed
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

