Novel 5-fluorouracil-resistant human esophageal squamous cell carcinoma cells with dihydropyrimidine dehydrogenase overexpression
- PMID: 26396918
- PMCID: PMC4568778
Novel 5-fluorouracil-resistant human esophageal squamous cell carcinoma cells with dihydropyrimidine dehydrogenase overexpression
Abstract
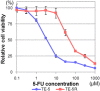
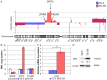
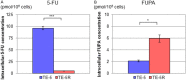
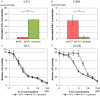
5-Fluorouracil (5-FU) is a key drug for the treatment of esophageal squamous cell carcinoma (ESCC); however, resistance to it remains a critical limitation to its clinical use. To clarify the mechanisms of 5-FU resistance of ESCC, we originally established 5-FU-resistant ESCC cells, TE-5R, by step-wise treatment with continuously increasing concentrations of 5-FU. The half maximal inhibitory concentration of 5-FU showed that TE-5R cells were 15.6-fold more resistant to 5-FU in comparison with parental TE-5 cells. TE-5R cells showed regional copy number amplification of chromosome 1p including the DPYD gene, as well as high mRNA and protein expressions of dihydropyrimidine dehydrogenase (DPD), an enzyme involved in 5-FU degradation. 5-FU treatment resulted in a significant decrease of the intracellular 5-FU concentration and increase of the concentration of α-fluoro-ureidopropionic acid (FUPA), a metabolite of 5-FU, in TE-5R compared with TE-5 cells in vitro. Conversely, gimeracil, a DPD inhibitor, markedly increased the intracellular 5-FU concentration, decreased the intracellular FUPA concentration, and attenuated 5-FU resistance of TE-5R cells. These results indicate that 5-FU resistance of TE-5R cells is due to the rapid degradation of 5-FU by DPD overexpression. The investigation of 5-FU-resistant ESCC with DPYD gene copy number amplification and consequent DPD overexpression may generate novel biological evidence to explore strategies against ESCC with 5-FU resistance.
Keywords: 5-fluorouracil; Esophageal squamous cell carcinoma; chemotherapy; dihydropyrimidine dehydrogenase; drug resistance.
Figures
References
-
- Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–412. - PubMed
-
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. - PubMed
-
- Ancona E, Ruol A, Santi S, Merigliano S, Sileni VC, Koussis H, Zaninotto G, Bonavina L, Peracchia A. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer. 2001;91:2165–2174. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
