Myeloperoxidase Nuclear Imaging for Epileptogenesis
- PMID: 26397127
- PMCID: PMC4770943
- DOI: 10.1148/radiol.2015141922
Myeloperoxidase Nuclear Imaging for Epileptogenesis
Erratum in
-
Myeloperoxidase Nuclear Imaging for Epileptogenesis.Radiology. 2017 Feb;282(2):614. doi: 10.1148/radiol.2017164044. Radiology. 2017. PMID: 28099101 Free PMC article. No abstract available.
Abstract
Purpose: To determine if myeloperoxidase (MPO) is involved in epileptogenesis and if molecular nuclear imaging can be used to noninvasively map inflammatory changes in epileptogenesis.
Materials and methods: The animal and human studies were approved by the institutional review boards. Pilocarpine-induced epileptic mice were treated with 4-aminobenzoic acid hydrazide (n = 46), a specific irreversible MPO inhibitor, or saline (n = 42). Indium-111-bis-5-hydroxytryptamide-diethylenetriaminepentaacetate was used to image brain MPO activity (n = 6 in the 4-aminobenzoic acid hydrazide and saline groups; n = 5 in the sham group) by using single photon emission computed tomography/computed tomography. The role of MPO in the development of spontaneous recurrent seizures was assessed by means of clinical symptoms and biochemical and histopathologic data. Human brain specimens from a patient with epilepsy and a patient without epilepsy were stained for MPO. The Student t test, one-way analysis of variance, and Mann-Whitney and Kruskal-Wallis tests were used. Differences were regarded as significant if P was less than .05.
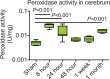
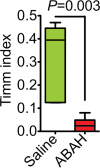
Results: MPO and leukocytes increased in the brain during epileptogenesis (P < .05). Blocking MPO delayed spontaneous recurrent seizures (99.6 vs 142 hours, P = .016), ameliorated the severity of spontaneous recurrent seizures (P < .05), and inhibited mossy fiber sprouting (Timm index, 0.31 vs 0.03; P = .003). Matrix metalloproteinase activity was upregulated during epileptogenesis in an MPO-dependent manner (1.44 vs 0.94 U/mg, P = .049), suggesting that MPO acts upstream of matrix metalloproteinases. MPO activity was mapped during epileptogenesis in vivo in the hippocampal regions. Resected temporal lobe tissue from a human patient with refractory epilepsy but not the temporal lobe tissue from a patient without seizures demonstrated positive MPO immunostaining, suggesting high translational potential for this imaging technology.
Conclusion: The findings of this study highlight an important role for MPO in epileptogenesis and show MPO to be a potential therapeutic target and imaging biomarker for epilepsy.
Figures










References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

