Neuroprotective Potential of Mesenchymal Stem Cell-Based Therapy in Acute Stages of TNBS-Induced Colitis in Guinea-Pigs
- PMID: 26397368
- PMCID: PMC4580595
- DOI: 10.1371/journal.pone.0139023
Neuroprotective Potential of Mesenchymal Stem Cell-Based Therapy in Acute Stages of TNBS-Induced Colitis in Guinea-Pigs
Abstract
Background & aims: The therapeutic benefits of mesenchymal stem cells (MSCs), such as homing ability, multipotent differentiation capacity and secretion of soluble bioactive factors which exert neuroprotective, anti-inflammatory and immunomodulatory properties, have been attributed to attenuation of autoimmune, inflammatory and neurodegenerative disorders. In this study, we aimed to determine the earliest time point at which locally administered MSC-based therapies avert enteric neuronal loss and damage associated with intestinal inflammation in the guinea-pig model of colitis.
Methods: At 3 hours after induction of colitis by 2,4,6-trinitrobenzene-sulfonate (TNBS), guinea-pigs received either human bone marrow-derived MSCs, conditioned medium (CM), or unconditioned medium by enema into the colon. Colon tissues were collected 6, 24 and 72 hours after administration of TNBS. Effects on body weight, gross morphological damage, immune cell infiltration and myenteric neurons were evaluated. RT-PCR, flow cytometry and antibody array kit were used to identify neurotrophic and neuroprotective factors released by MSCs.
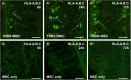
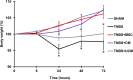
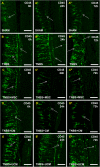
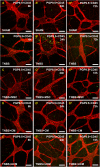
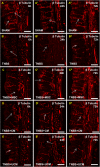
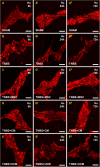
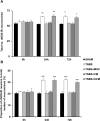
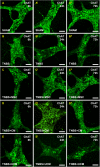
Results: MSC and CM treatments prevented body weight loss, reduced infiltration of leukocytes into the colon wall and the myenteric plexus, facilitated repair of damaged tissue and nerve fibers, averted myenteric neuronal loss, as well as changes in neuronal subpopulations. The neuroprotective effects of MSC and CM treatments were observed as early as 24 hours after induction of inflammation even though the inflammatory reaction at the level of the myenteric ganglia had not completely subsided. Substantial number of neurotrophic and neuroprotective factors released by MSCs was identified in their secretome.
Conclusion: MSC-based therapies applied at the acute stages of TNBS-induced colitis start exerting their neuroprotective effects towards enteric neurons by 24 hours post treatment. The neuroprotective efficacy of MSC-based therapies can be exerted independently to their anti-inflammatory effects.
Conflict of interest statement
Figures




References
-
- Farrell D, Savage E. Symptom burden: a forgotten area of measurement in inflammatory bowel disease. Int J Nurs Prac. 2012;18: 497–500. - PubMed
-
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369: 1641–57. - PubMed
-
- Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369: 1627–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

