The Eltrombopag antitumor effect on hepatocellular carcinoma
- PMID: 26397763
- PMCID: PMC4599203
- DOI: 10.3892/ijo.2015.3180
The Eltrombopag antitumor effect on hepatocellular carcinoma
Abstract
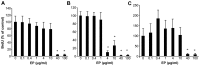
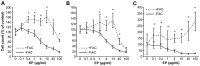
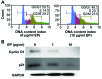
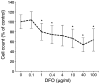
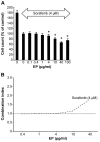
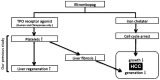
Currently, sorafenib is the only available chemotherapeutic agent for advanced hepatocellular carcinoma (HCC), but it cannot be used in patients with liver cirrhosis (LC) or thrombocytopenia. In these cases, sorafenib is likely effective if given in combination with treatments that increase the number of platelets, such as thrombopoietin (TPO) receptor agonists. Increasing the platelet count via TPO treatment resulted in reduction of LC. Eltrombopag (EP), a TPO receptor agonist, has been reported to have antitumor effects against certain cancers, despite their lack of TPO receptor expression. We hypothesized that EP may possess antitumor activity against HCC in addition to its ability to suppress hepatic fibrosis by increasing the platelet count. In the present study, the antitumor activity of EP was examined by assessing the inhibition of cell proliferation and then ascertaining the ability of iron supplementation to reverse these effects in HepG2, Hep3B and Huh7 cells. In addition, a cell cycle assay was performed using flow cytometry, and signal transduction was evaluated by analyzing cell cycle-related protein expression. The results of EP were compared with those of the most common iron chelator, deferoxamine (DFO). The combined effect of EP and sorafenib was also assessed. The results revealed that EP exerts antitumor activity in HCC that is mediated by the modulation of intracellular iron content. EP suppressed the expression of the cell cycle-related protein cyclin D1 and elicited cell cycle arrest in the G0/G1 phase. The activity of EP was comparable to that of DFO in HCC, and EP did not compete with sorafenib at low concentrations. In conclusion, our findings suggest that EP is a good candidate chemotherapeutic agent for the treatment of HCC in patients with LC and thrombocytopenia.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

