Letrozole, Gonadotropin, or Clomiphene for Unexplained Infertility
- PMID: 26398071
- PMCID: PMC4739644
- DOI: 10.1056/NEJMoa1414827
Letrozole, Gonadotropin, or Clomiphene for Unexplained Infertility
Abstract
Background: The standard therapy for women with unexplained infertility is gonadotropin or clomiphene citrate. Ovarian stimulation with letrozole has been proposed to reduce multiple gestations while maintaining live birth rates.
Methods: We enrolled couples with unexplained infertility in a multicenter, randomized trial. Ovulatory women 18 to 40 years of age with at least one patent fallopian tube were randomly assigned to ovarian stimulation (up to four cycles) with gonadotropin (301 women), clomiphene (300), or letrozole (299). The primary outcome was the rate of multiple gestations among women with clinical pregnancies.
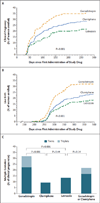
Results: After treatment with gonadotropin, clomiphene, or letrozole, clinical pregnancies occurred in 35.5%, 28.3%, and 22.4% of cycles, and live birth in 32.2%, 23.3%, and 18.7%, respectively; pregnancy rates with letrozole were significantly lower than the rates with standard therapy (gonadotropin or clomiphene) (P=0.003) or gonadotropin alone (P<0.001) but not with clomiphene alone (P=0.10). Among ongoing pregnancies with fetal heart activity, the multiple gestation rate with letrozole (9 of 67 pregnancies, 13%) did not differ significantly from the rate with gonadotropin or clomiphene (42 of 192, 22%; P=0.15) or clomiphene alone (8 of 85, 9%; P=0.44) but was lower than the rate with gonadotropin alone (34 of 107, 32%; P=0.006). All multiple gestations in the clomiphene and letrozole groups were twins, whereas gonadotropin treatment resulted in 24 twin and 10 triplet gestations. There were no significant differences among groups in the frequencies of congenital anomalies or major fetal and neonatal complications.
Conclusions: In women with unexplained infertility, ovarian stimulation with letrozole resulted in a significantly lower frequency of multiple gestation but also a lower frequency of live birth, as compared with gonadotropin but not as compared with clomiphene. (Funded by the National Institutes of Health and others; ClinicalTrials.gov number, NCT01044862.).
Figures
Comment in
-
Fewer live births occur with letrozole than standard therapy for unexplained infertility, study shows.BMJ. 2015 Sep 24;351:h5070. doi: 10.1136/bmj.h5070. BMJ. 2015. PMID: 26407841 No abstract available.
-
Therapy: Unexplained infertility - ongoing transatlantic debate.Nat Rev Endocrinol. 2016 Jan;12(1):8-10. doi: 10.1038/nrendo.2015.200. Epub 2015 Nov 20. Nat Rev Endocrinol. 2016. PMID: 26585663 No abstract available.
References
-
- McClamrock HD, Jones HW, Jr, Adashi EY. Ovarian stimulation and intrauterine insemination at the quarter centennial: implications for the multiple births epidemic. Fertil Steril. 2012;97:802–809. - PubMed
-
- Ghesquiere SL, Castelain EG, Spies-sens C, Meuleman CL, D’Hooghe TM. Relationship between follicle number and (multiple) live birth rate after controlled ovarian hyperstimulation and intrauter-ine insemination. Am J Obstet Gynecol. 2007;197(4):589.e1–589.e5. - PubMed
-
- Samani FG, Farzadi L, Nezami N, Tar-zamni MK, Soleimani F. Endometrial and follicular development following letro-zole intervention in unexplained infertile patients failed to get pregnant with clo-miphene citrate. Arch Gynecol Obstet. 2009;280:201–205. - PubMed
-
- Fisch P, Casper RF, Brown SE, et al. Unexplained infertility: evaluation of treatment with clomiphene citrate and human chorionic gonadotropin. Fertil Steril. 1989;51:828–833. - PubMed
-
- Practice Committee of American Society for Reproductive Medicine. Multiple gestation associated with infertility therapy: an American Society for Reproductive Medicine Practice Committee opinion. Fertil Steril. 2012;97:825–834. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HD039005/HD/NICHD NIH HHS/United States
- U10 HD077680/HD/NICHD NIH HHS/United States
- U10 HD27049/HD/NICHD NIH HHS/United States
- U10 HD055936/HD/NICHD NIH HHS/United States
- U10 HD055925/HD/NICHD NIH HHS/United States
- R01 HD029834/HD/NICHD NIH HHS/United States
- U10 HD027049/HD/NICHD NIH HHS/United States
- UL1 TR002014/TR/NCATS NIH HHS/United States
- U10 HD055942/HD/NICHD NIH HHS/United States
- UL1 TR000127/TR/NCATS NIH HHS/United States
- U10 HD38998/HD/NICHD NIH HHS/United States
- U10 HD038998/HD/NICHD NIH HHS/United States
- U54 HD028934/HD/NICHD NIH HHS/United States
- U10 HD055944/HD/NICHD NIH HHS/United States
- U10 HD39005/HD/NICHD NIH HHS/United States
- U10 HD038992/HD/NICHD NIH HHS/United States
- U10 U54-HD29834/HD/NICHD NIH HHS/United States
- U10 HD38992/HD/NICHD NIH HHS/United States
- P50 HD028934/HD/NICHD NIH HHS/United States
- U10HD055925/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
