Intensified Chemotherapy With Dexrazoxane Cardioprotection in Newly Diagnosed Nonmetastatic Osteosarcoma: A Report From the Children's Oncology Group
- PMID: 26398490
- PMCID: PMC4779061
- DOI: 10.1002/pbc.25753
Intensified Chemotherapy With Dexrazoxane Cardioprotection in Newly Diagnosed Nonmetastatic Osteosarcoma: A Report From the Children's Oncology Group
Abstract
Background: Although chemotherapy has improved outcome of osteosarcoma, 30-40% of patients succumb to this disease. Survivors experience substantial morbidity and mortality from anthracycline-induced cardiotoxicity. We hypothesized that the cardioprotectant dexrazoxane would (i) support escalation of the cumulative doxorubicin dose (600 mg/m(2)) and (ii) not interfere with the cytotoxicity of chemotherapy measured by necrosis grading in comparison to historical control data.
Procedure: Children and adolescents with nonmetastatic osteosarcoma were treated with MAP (methotrexate, doxorubicin, cisplatin) or MAPI (MAP/ifosfamide). Dexrazoxane was administered with all doxorubicin doses. Cardioprotection was assessed by measuring left ventricular fractional shortening. Interference with chemotherapy-induced cytotoxicity was determined by measuring tumor necrosis after induction chemotherapy. Feasibility of intensifying therapy with either high cumulative-dose doxorubicin or high-dose ifosfamide/etoposide was evaluated for "standard responders" (SR, <98% tumor necrosis at definitive surgery).
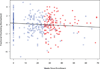
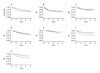
Results: Dexrazoxane did not compromise response to induction chemotherapy. With doxorubicin (450-600 mg/m(2)) and dexrazoxane, grade 1 or 2 left ventricular dysfunction occurred in five patients; 4/5 had transient effects. Left ventricular fractional shortening z-scores (FSZ) showed minimal reductions (0.0170 ± 0.009/week) over 78 weeks. Two patients (<1%) had secondary leukemia, one as a first event, a similar rate to what has been observed in prior trials. Intensification with high-dose ifosfamide/etoposide was also feasible.
Conclusions: Dexrazoxane cardioprotection was safely administered. It did not impair tumor response or increase the risk of secondary malignancy. Dexrazoxane allowed for therapeutic intensification increasing the cumulative doxorubicin dose in SR to induction chemotherapy. These findings support the use of dexrazoxane in children and adolescents with osteosarcoma.
Keywords: dexrazoxane cardioprotection; intensified chemotherapy; newly diagnosed; nonmetastatic osteosarcoma.
© 2015 Wiley Periodicals, Inc.
Figures

References
-
- Goorin AM, Schwartzentruber DJ, Devidas M, Gebhart MC, Ayala AG, Harris MB, Helman LJ, Grier HE, Link MP. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;21:1574–1580. - PubMed
-
- Meyers PA, Schwartz CL, Krailo MD, Healy JH, Berstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM, Harris M, Kleinerman E, Link MP, Nadel H, Nieder M, Siegal GP, Weiner MA, Wells RJ, Womer RB, Grier HE. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival – a report from the Children’s Oncology Group. J Clin Oncol. 2008;26:633–638. - PubMed
-
- Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol. 1987;5:21–26. - PubMed
-
- Ferrari S, Smeland S, Mercuri M, Healy JH, Berstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM, Harris M, Kleinerman E, Link MP, Nadel H, Nieder M, Siegal GP, Weiner MA, Wells RJ, Womer RB, Grier HE. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23:8845–8852. - PubMed
-
- Bacci G, Longhi A, Fagioli F, Briccoli A, Versari M, Picci P. Adjuvant and neoadjuvant chemotherapy for osteosarcoma of the extremities: 27 year experience at Rizzoli Institute, Italy. Eur J Cancer. 2005;41:2836–2845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

