Isolation of a Novel Phage with Activity against Streptococcus mutans Biofilms
- PMID: 26398909
- PMCID: PMC4580409
- DOI: 10.1371/journal.pone.0138651
Isolation of a Novel Phage with Activity against Streptococcus mutans Biofilms
Abstract
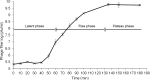
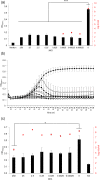
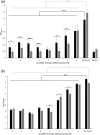
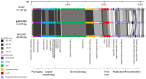
Streptococcus mutans is one of the principal agents of caries formation mainly, because of its ability to form biofilms at the tooth surface. Bacteriophages (phages) are promising antimicrobial agents that could be used to prevent or treat caries formation by S. mutans. The aim of this study was to isolate new S. mutans phages and to characterize their antimicrobial properties. A new phage, ɸAPCM01, was isolated from a human saliva sample. Its genome was closely related to the only two other available S. mutans phage genomes, M102 and M102AD. ɸAPCM01 inhibited the growth of S. mutans strain DPC6143 within hours in broth and in artificial saliva at multiplicity of infections as low as 2.5x10-5. In the presence of phage ɸAPCM01 the metabolic activity of a S. mutans biofilm was reduced after 24 h of contact and did not increased again after 48 h, and the live cells in the biofilm decreased by at least 5 log cfu/ml. Despite its narrow host range, this newly isolated S. mutans phage exhibits promising antimicrobial properties.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

