Glycolytic inhibitor 2-deoxyglucose simultaneously targets cancer and endothelial cells to suppress neuroblastoma growth in mice
- PMID: 26398947
- PMCID: PMC4610240
- DOI: 10.1242/dmm.021667
Glycolytic inhibitor 2-deoxyglucose simultaneously targets cancer and endothelial cells to suppress neuroblastoma growth in mice
Abstract
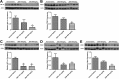
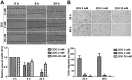
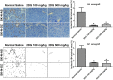
Neuroblastoma is characterized by a wide range of clinical manifestations and associated with poor prognosis when there is amplification of MYCN oncogene or high expression of Myc oncoproteins. In a previous in vitro study, we found that the glycolytic inhibitor 2-deoxyglucose (2DG) could suppress the growth of neuroblastoma cells, particularly in those with MYCN amplification. In this study, we established a mouse model of neuroblastoma xenografts with SK-N-DZ and SK-N-AS cells treated with 2DG by intraperitoneal injection twice a week for 3 weeks at 100 or 500 mg/kg body weight. We found that 2DG was effective in suppressing the growth of both MYCN-amplified SK-N-DZ and MYCN-non-amplified SK-N-AS neuroblastoma xenografts, which was associated with downregulation of HIF-1α, PDK1 and c-Myc, and a reduction in the number of tumor blood vessels. In vitro study showed that 2DG can suppress proliferation, cause apoptosis and reduce migration of murine endothelial cells, with inhibition of the formation of lamellipodia and filopodia and disorganization of F-actin filaments. The results suggest that 2DG might simultaneously target cancer cells and endothelial cells in the neuroblastoma xenografts in mice regardless of the status of MYCN amplification, providing a potential therapeutic opportunity to use 2DG or other glycolytic inhibitors for the treatment of patients with refractory neuroblastoma.
Keywords: 2-deoxyglucose; Endothelial cell; MYCN amplification; Neuroblastoma; Xenograft.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures



References
-
- Chuang J.-H., Chou M.-H., Tai M.-H., Lin T.-K., Liou C.-W., Chen T., Hsu W.-M. and Wang P.-W. (2013). 2-Deoxyglucose treatment complements the cisplatin- or BH3-only mimetic-induced suppression of neuroblastoma cell growth. Int. J. Biochem. Cell Biol. 45, 944-951. 10.1016/j.biocel.2013.01.019 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

