Genetic associations with viral respiratory illnesses and asthma control in children
- PMID: 26399222
- PMCID: PMC4715666
- DOI: 10.1111/cea.12642
Genetic associations with viral respiratory illnesses and asthma control in children
Abstract
Background: Viral respiratory infections can cause acute wheezing illnesses in children and exacerbations of asthma.
Objective: We sought to identify variation in genes with known antiviral and pro-inflammatory functions to identify specific associations with more severe viral respiratory illnesses and the risk of virus-induced exacerbations during the peak fall season.
Methods: The associations between genetic variation at 326 SNPs in 63 candidate genes and 10 phenotypes related to viral respiratory infection and asthma control were examined in 226 children enrolled in the RhinoGen study. Replication of asthma control phenotypes was performed in 2128 children in the Copenhagen Prospective Study on Asthma in Childhood (COPSAC). Significant associations in RhinoGen were further validated using virus-induced wheezing illness and asthma phenotypes in an independent sample of 122 children enrolled in the Childhood Origins of Asthma (COAST) birth cohort study.
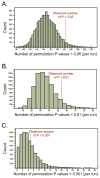
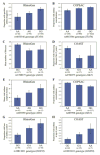
Results: A significant excess of P values smaller than 0.05 was observed in the analysis of the 10 RhinoGen phenotypes. Polymorphisms in 12 genes were significantly associated with variation in the four phenotypes showing a significant enrichment of small P values. Six of those genes (STAT4, JAK2, MX1, VDR, DDX58, and EIF2AK2) also showed significant associations with asthma exacerbations in the COPSAC study or with asthma or virus-induced wheezing phenotypes in the COAST study.
Conclusions: We identified genetic factors contributing to individual differences in childhood viral respiratory illnesses and virus-induced exacerbations of asthma. Defining mechanisms of these associations may provide insight into the pathogenesis of viral respiratory infections and virus-induced exacerbations of asthma.
Keywords: allergic sensitization; asthma; children; cold symptoms; genetic association; human rhinovirus; viral respiratory illness; wheezing.
© 2015 John Wiley & Sons Ltd.
Figures
References
-
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. - PubMed
-
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446. - PubMed
-
- Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, Carlson-Dakes KT, Salazar LP, DaSilva DF, Tisler CJ, Gern JE, Lemanske RF. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

