Inhibitory luminopsins: genetically-encoded bioluminescent opsins for versatile, scalable, and hardware-independent optogenetic inhibition
- PMID: 26399324
- PMCID: PMC4585826
- DOI: 10.1038/srep14366
Inhibitory luminopsins: genetically-encoded bioluminescent opsins for versatile, scalable, and hardware-independent optogenetic inhibition
Abstract
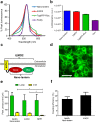
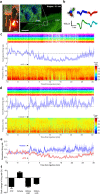
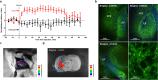
Optogenetic techniques provide an unprecedented ability to precisely manipulate neural activity in the context of complex neural circuitry. Although the toolbox of optogenetic probes continues to expand at a rapid pace with more efficient and responsive reagents, hardware-based light delivery is still a major hurdle that limits its practical use in vivo. We have bypassed the challenges of external light delivery by directly coupling a bioluminescent light source (a genetically encoded luciferase) to an inhibitory opsin, which we term an inhibitory luminopsin (iLMO). iLMO was shown to suppress action potential firing and synchronous bursting activity in vitro in response to both external light and luciferase substrate. iLMO was further shown to suppress single-unit firing rate and local field potentials in the hippocampus of anesthetized rats. Finally, expression of iLMO was scaled up to multiple structures of the basal ganglia to modulate rotational behavior of freely moving animals in a hardware-independent fashion. This novel class of optogenetic probes demonstrates how non-invasive inhibition of neural activity can be achieved, which adds to the versatility, scalability, and practicality of optogenetic applications in freely behaving animals.
Figures


References
-
- AM A. et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 4, S143–56 (2007). - PubMed
-
- Yizhar O., Fenno L. E., Davidson T. J., Mogri M. & Deisseroth K. Optogenetics in neural systems. Neuron 71, 9–34 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

