Optimization of neuronal cultures from rat superior cervical ganglia for dual patch recording
- PMID: 26399440
- PMCID: PMC4585864
- DOI: 10.1038/srep14455
Optimization of neuronal cultures from rat superior cervical ganglia for dual patch recording
Abstract
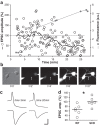
Superior cervical ganglion neurons (SCGN) are often used to investigate neurotransmitter release mechanisms. In this study, we optimized the dissociation and culture conditions of rat SCGN cultures for dual patch clamp recordings. Two weeks in vitro are sufficient to achieve a significant CNTF-induced cholinergic switch and to develop mature and healthy neuronal profiles suited for detailed patch clamp analysis. One single pup provides sufficient material to prepare what was formerly obtained from 12 to 15 animals. The suitability of these cultures to study neurotransmitter release mechanisms was validated by presynaptically perturbing the interaction of the v-SNARE VAMP2 with the vesicular V-ATPase V0c subunit.
Figures



References
-
- Plummer M. R., Logothetis D. E. & Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron 2, 1453–63 (1989). - PubMed
-
- Vivas O., Castro H., Arenas I., Elias-Vinas D. & Garcia D. E. PIP2 hydrolysis is responsible for voltage independent inhibition of CaV2.2 channels in sympathetic neurons. Biochemical and Biophysical Research Communications 432, 275–280 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

