A Cluster-Randomized Trial to Evaluate a Mother-Daughter Dyadic Educational Intervention for Increasing HPV Vaccination Coverage in American Indian Girls
- PMID: 26399648
- PMCID: PMC4779421
- DOI: 10.1007/s10900-015-0093-2
A Cluster-Randomized Trial to Evaluate a Mother-Daughter Dyadic Educational Intervention for Increasing HPV Vaccination Coverage in American Indian Girls
Abstract
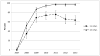
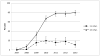
We evaluated whether delivering educational presentations on human papillomavirus (HPV) to American Indian mothers affected HPV vaccination rates in their adolescent daughters. In March-April 2012, we recruited Hopi mothers or female guardians with daughters aged 9-12 years for a cluster-randomized intervention study on the Hopi Reservation. Participants attended mother-daughter dinners featuring educational presentations for mothers on either HPV (intervention) or juvenile diabetes (control) and completed baseline surveys. Eleven months later, we surveyed mothers on their daughters' HPV vaccine uptake. We also reviewed aggregated immunization reports from the Indian Health Service to assess community-level HPV vaccination coverage from 2007 to 2013. Ninety-seven mother-daughter dyads participated; nine mothers reported that their daughters completed the three-dose HPV vaccination series before recruitment. Among the remaining mothers, 63 % completed the follow-up survey. Adjusting for household income, the proportion of daughters completing vaccination within 11 months post-intervention was similar in the intervention and control groups (32 vs. 28 %, adjusted RR = 1.2, 95 % confidence interval (CI) 0.6-2.3). Among unvaccinated daughters, those whose mothers received HPV education were more likely to initiate vaccination (50 vs. 27 %, adjusted RR = 2.6, 95 % CI 1.4-4.9) and complete three doses (adjusted RR = 4.0, 95 % CI 1.2-13.1) than girls whose mothers received diabetes education. Community-level data showed that 80 % of girls aged 13-17 years and 20 % of girls aged 11-12 completed the vaccination series by 2013. HPV vaccine uptake in Hopi girls aged 13-17 years is significantly higher than the U.S. national average. Brief educational presentations on HPV delivered to American Indian mothers might increase HPV vaccination rates in daughters aged 9-12 years.
Keywords: American Indian/Alaska Native (AI/AN); Education; Human papillomavirus (HPV); Intervention; Vaccination.
Conflict of interest statement
Figures
References
-
- Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–23. - PubMed
-
- Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2014;63(RR-05):1–30. - PubMed
-
- Munoz N, Bosch FX, Castellsague X, Diaz M, De Sanjose S, Hammouda D, et al. Against which human papillomavirus types shall we vaccinate and screen? the international perspective. Int J Cancer. 2004;111(2):278–285. - PubMed
-
- Bryan JT. Developing an HPV vaccine to prevent cervical cancer and genital warts. Vaccine. 2007;25(16):3001–3006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

