The RUNX complex: reaching beyond haematopoiesis into immunity
- PMID: 26399680
- PMCID: PMC4693896
- DOI: 10.1111/imm.12535
The RUNX complex: reaching beyond haematopoiesis into immunity
Abstract
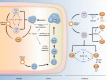
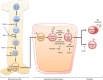
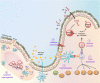
Among their diverse roles as transcriptional regulators during development and cell fate specification, the RUNX transcription factors are best known for the parts they play in haematopoiesis. RUNX proteins are expressed throughout all haematopoietic lineages, being necessary for the emergence of the first haematopoietic stem cells to their terminal differentiation. Although much progress has been made since their discoveries almost two decades ago, current appreciation of RUNX in haematopoiesis is largely grounded in their lineage-specifying roles. In contrast, the importance of RUNX to immunity has been mostly obscured for historic, technical and conceptual reasons. However, this paradigm is likely to shift over time, as a primary purpose of haematopoiesis is to resource the immune system. Furthermore, recent evidence suggests a role for RUNX in the innate immunity of non-haematopoietic cells. This review takes a haematopoiesis-centric approach to collate what is known of RUNX's contribution to the overall mammalian immune system and discuss their growing prominence in areas such as autoimmunity, inflammatory diseases and mucosal immunity.
Keywords: RUNX transcription factors; autoimmunity; haematopoiesis; immune system; mucosal immunity.
© 2015 John Wiley & Sons Ltd.
Figures
References
-
- Ito Y, Bae SC, Chuang LS. The RUNX family: developmental regulators in cancer. Nat Rev Cancer 2015; 15:81–95. - PubMed
-
- Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 1996; 84:321–30. - PubMed
-
- Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res 2010; 339:189–95. - PubMed
-
- de Bruijn MF, Speck NA. Core‐binding factors in hematopoiesis and immune function. Oncogene 2004; 23:4238–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

