Antigen-B Cell Receptor Complexes Associate with Intracellular major histocompatibility complex (MHC) Class II Molecules
- PMID: 26400081
- PMCID: PMC4646406
- DOI: 10.1074/jbc.M115.649582
Antigen-B Cell Receptor Complexes Associate with Intracellular major histocompatibility complex (MHC) Class II Molecules
Abstract
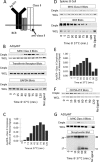
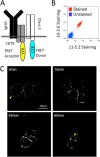
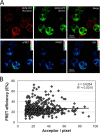
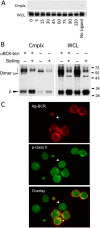
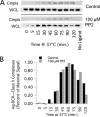
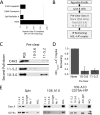
Antigen processing and MHC class II-restricted antigen presentation by antigen-presenting cells such as dendritic cells and B cells allows the activation of naïve CD4+ T cells and cognate interactions between B cells and effector CD4+ T cells, respectively. B cells are unique among class II-restricted antigen-presenting cells in that they have a clonally restricted antigen-specific receptor, the B cell receptor (BCR), which allows the cell to recognize and respond to trace amounts of foreign antigen present in a sea of self-antigens. Moreover, engagement of peptide-class II complexes formed via BCR-mediated processing of cognate antigen has been shown to result in a unique pattern of B cell activation. Using a combined biochemical and imaging/FRET approach, we establish that internalized antigen-BCR complexes associate with intracellular class II molecules. We demonstrate that the M1-paired MHC class II conformer, shown previously to be critical for CD4 T cell activation, is incorporated selectively into these complexes and loaded selectively with peptide derived from BCR-internalized cognate antigen. These results demonstrate that, in B cells, internalized antigen-BCR complexes associate with intracellular MHC class II molecules, potentially defining a site of class II peptide acquisition, and reveal a selective role for the M1-paired class II conformer in the presentation of cognate antigen. These findings provide key insights into the molecular mechanisms used by B cells to control the source of peptides charged onto class II molecules, allowing the immune system to mount an antibody response focused on BCR-reactive cognate antigen.
Keywords: B cell receptor (BCR); FRET; MHC; antigen presentation; antigen processing; antigen-presenting cell (APC); imaging; lymphocyte.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Hulpke S., and Tampé R. (2013) The MHC I loading complex: a multitasking machinery in adaptive immunity. Trends Biochem. Sci. 38, 412–420 - PubMed
-
- Chesnut R. W., and Grey H. M. (1985) Antigen presenting cells and mechanisms of antigen presentation. Crit. Rev. Immunol. 5, 263–316 - PubMed
-
- Chesnut R. W., and Grey H. M. (1986) Antigen presentation by B cells and its significance in T-B interactions. Adv. Immunol. 39, 51–94 - PubMed
-
- Lanzavecchia A. (1987) Antigen uptake and accumulation in antigen-specific B cells. Immunol. Rev. 99, 39–51 - PubMed
-
- Nashar T. O., and Drake J. R. (2005) The pathway of antigen uptake and processing dictates MHC class II-mediated B cell survival and activation. J. Immunol. 174, 1306–1316 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

