Towards aspirin-inspired self-immolating molecules which target the cyclooxygenases
- PMID: 26400105
- PMCID: PMC4644089
- DOI: 10.1039/c5ob01805f
Towards aspirin-inspired self-immolating molecules which target the cyclooxygenases
Abstract
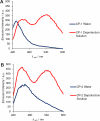
Cyclooxygenases (COXs) are enzymes that play a vital role in the inflammatory cascade through the generation of prostaglandins. Their over-expression has been implicated in numerous diseases. In particular, over-expression of COX-2 has been shown to be a predictive biomarker for progression of pre-malignant lesions towards invasive cancer in various tissues. This makes the early detection of COX-2 expressing lesions of high clinical relevance. Herein we describe the development of the first self-immolating trigger which targets COXs. We incorporated our trigger design into 2 activatable fluorogenic probes and demonstrated COX-specific activation in vitro. Experimental data revealed probe activation was likely caused by solvent-exposed amino acids on the surface of the COXs. Overall, the probes reported here mark the first step towards developing self-immolating imaging/therapeutic agents targeted to specific COXs.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

