Learning to balance on one leg: motor strategy and sensory weighting
- PMID: 26400255
- PMCID: PMC4737414
- DOI: 10.1152/jn.00434.2015
Learning to balance on one leg: motor strategy and sensory weighting
Abstract
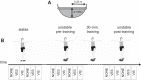
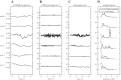
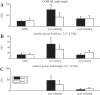
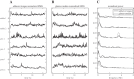
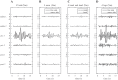
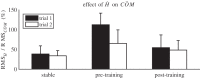
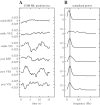
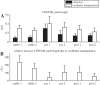
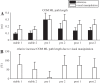
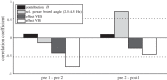
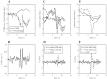
We investigated motor and sensory changes underlying learning of a balance task. Fourteen participants practiced balancing on one leg on a board that could freely rotate in the frontal plane. They performed six, 16-s trials standing on one leg on a stable surface (2 trials without manipulation, 2 with vestibular, and 2 with visual stimulation) and six trials on the balance board before and after a 30-min training. Center of mass (COM) movement, segment, and total angular momenta and board angles were determined. Trials on stable surface were compared with trials after training to assess effects of surface conditions. Trials pretraining and posttraining were compared to assess rapid (between trials pretraining) and slower (before and after training) learning, and sensory manipulation trials were compared with unperturbed trials to assess sensory weighting. COM excursions were larger on the unstable surface but decreased with practice, with the largest improvement over the pretraining trials. Changes in angular momentum contributed more to COM acceleration on the balance board, but with practice this decreased. Visual stimulation increased sway similarly in both surface conditions, while vestibular stimulation increased sway less on the balance board. With practice, the effects of visual and vestibular stimulation increased rapidly. Initially, oscillations of the balance board occurred at 3.5 Hz, which decreased with practice. The initial decrease in sway with practice was associated with upweighting of visual information, while later changes were associated with suppression of oscillations that we suggest are due to too high proprioceptive feedback gains.
Keywords: motor learning; postural control; sensory integration; sway.
Copyright © 2015 the American Physiological Society.
Figures


References
-
- Andreopoulou G, Maaswinkel E, Cofre Lizama LE, van Dieen JH. Effects of support surface stability on feedback control of trunk posture. Exp Brain Res 233: 1079–1087, 2015. - PubMed
-
- Asseman FB, Caron O, Crémieux J. Are there specific conditions for which expertise in gymnastics could have an effect on postural control and performance? Gait Posture 27: 76–81, 2008. - PubMed
-
- Chalmers GR, Knutzen KM. Soleus H-reflex gain in healthy elderly and young adults when lying, standing, and balancing. J Gerontol A Biol Sci Med Sci 57: B321–B329, 2002. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

