Trends in utilization of FDA expedited drug development and approval programs, 1987-2014: cohort study
- PMID: 26400751
- PMCID: PMC4580726
- DOI: 10.1136/bmj.h4633
Trends in utilization of FDA expedited drug development and approval programs, 1987-2014: cohort study
Abstract
Objective: To evaluate the use of special expedited development and review pathways at the US Food and Drug Administration over the past two decades.
Design: Cohort study.
Setting: FDA approved novel therapeutics between 1987 and 2014.
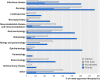
Population: Publicly available sources provided each drug's year of approval, their innovativeness (first in class versus not first in class), World Health Organization Anatomic Therapeutic Classification, and which (if any) of the FDA's four primary expedited development and review programs or designations were associated with each drug: orphan drug, fast track, accelerated approval, and priority review.
Main outcome measures: Logistic regression models evaluated trends in the proportion of drugs associated with each of the four expedited development and review programs. To evaluate the number of programs associated with each approved drug over time, Poisson models were employed, with the number of programs as the dependent variable and a linear term for year of approval. The difference in trends was compared between drugs that were first in class and those that were not.
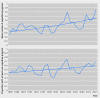
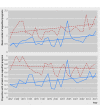
Results: The FDA approved 774 drugs during the study period, with one third representing first in class agents. Priority review (43%) was the most prevalent of the four programs, with accelerated approval (9%) the least common. There was a significant increase of 2.6% per year in the number of expedited review and approval programs granted to each newly approved agent (incidence rate ratio 1.026, 95% confidence interval 1.017 to 1.035, P<0.001), and a 2.4% increase in the proportion of drugs associated with at least one such program (odds ratio 1.024, 95% confidence interval 1.006 to 1.043, P=0.009). Driving this trend was an increase in the proportion of approved, non-first in class drugs associated with at least one program for drugs (P=0.03 for interaction).
Conclusions: In the past two decades, drugs newly approved by the FDA have been associated with an increasing number of expedited development or review programs. Though expedited programs should be strictly limited to drugs providing noticeable clinical advances, this trend is being driven by drugs that are not first in class and thus potentially less innovative.
© Kesselheim et al 2015.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
References
-
- Food and Drug Administration. Phases of an investigation: phase 1. 21 CFR § 312.21(a). FDA, 2014.
-
- Food and Drug Administration. Phases of an investigation: phase 2. 21 CFR § 312.21(b). FDA, 2014.
-
- Food and Drug Administration. Phases of an investigation: phase 3. 21 CFR § 312.21(c). FDA, 2014.
-
- Darrow JJ, Avorn J, Kesselheim AS. New FDA breakthrough-drug category—implications for patients. N Engl J Med 2014;370:1252-8. - PubMed
-
- Designation of Drugs for Rare Diseases or Conditions, 21 U.S.C. § 360bb(a)(2) (2008).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
