The Effect of Platelet-Rich Plasma in Hair Regrowth: A Randomized Placebo-Controlled Trial
- PMID: 26400925
- PMCID: PMC4622412
- DOI: 10.5966/sctm.2015-0107
The Effect of Platelet-Rich Plasma in Hair Regrowth: A Randomized Placebo-Controlled Trial
Abstract
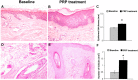
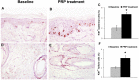
Platelet-rich plasma (PRP) has emerged as a new treatment modality in regenerative plastic surgery, and preliminary evidence suggests that it might have a beneficial role in hair regrowth. Here, we report the results of a randomized, evaluator-blinded, placebo-controlled, half-head group study to compare, with the aid of computerized trichograms, hair regrowth with PRP versus placebo. The safety and clinical efficacy of autologous PRP injections for pattern hair loss were investigated. PRP, prepared from a small volume of blood, was injected on half of the selected patients' scalps with pattern hair loss. The other half was treated with placebo. Three treatments were administered to each patient at 30-day intervals. The endpoints were hair regrowth, hair dystrophy as measured by dermoscopy, burning or itching sensation, and cell proliferation as measured by Ki67 evaluation. Patients were followed for 2 years. Of the 23 patients enrolled, 3 were excluded. At the end of the 3 treatment cycles, the patients presented clinical improvement in the mean number of hairs, with a mean increase of 33.6 hairs in the target area, and a mean increase in total hair density of 45.9 hairs per cm² compared with baseline values. No side effects were noted during treatment. Microscopic evaluation showed the increase of epidermis thickness and of the number of hair follicles 2 weeks after the last PRP treatment compared with baseline value (p < .05). We also observed an increase of Ki67(+) keratinocytes in the epidermis and of hair follicular bulge cells, and a slight increase of small blood vessels around hair follicles in the treated skin compared with baseline (p < .05). Relapse of androgenic alopecia was not evaluated in all patients until 12 months after the last treatment. After 12 months, 4 patients reported progressive hair loss; this was more evident 16 months after the last treatment. Those four patients were re-treated. Our data clearly highlight the positive effects of PRP injections on male pattern hair loss and absence of major side effects. PRP may serve as a safe and effective treatment option against hair loss; more extensive controlled studies are needed.
Significance: Platelet-rich plasma (PRP) has emerged as a new treatment modality in regenerative plastic surgery, and preliminary evidence suggests that it might have a beneficial role in hair regrowth. Here, the results of a randomized, placebo-controlled, half-head group study to compare the hair regrowth with PRP versus placebo are reported. Hair regrowth was quantified by a blinded evaluator using computerized trichograms. The safety and clinical efficacy of autologous PRP injections for pattern hair loss were investigated. Of the 23 patients enrolled, 3 were excluded. At the end of the 3 treatment cycles, the patients presented clinical improvement in the mean number of hairs, with a mean increase of 33.6 hairs in the target area and a mean increase in total hair density of 45.9 hairs per cm² compared with baseline values. No side effects were noted during treatment. The data clearly highlight the positive effects of PRP injections on male pattern hair loss and absence of major side effects. PRP may serve as a safe and effective treatment option against hair loss; more extensive controlled studies are needed.
Keywords: Aging; Autologous; Clinical translations; Clinical trials.
©AlphaMed Press.
Figures



References
-
- Li ZJ, Choi HI, Choi DK, et al. Autologous platelet-rich plasma: A potential therapeutic tool for promoting hair growth. Dermatol Surg. 2012;38:1040–1046. - PubMed
-
- Trink A, Sorbellini E, Bezzola P, et al. A randomized, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata. Br J Dermatol. 2013;169:690–694. - PubMed
-
- Uebel CO, da Silva JB, Cantarelli D, et al. The role of platelet plasma growth factors in male pattern baldness surgery. Plast Reconstr Surg. 2006;118:1458–1466; discussion 1467. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

