Amygdala Signaling during Foraging in a Hazardous Environment
- PMID: 26400931
- PMCID: PMC4579372
- DOI: 10.1523/JNEUROSCI.0407-15.2015
Amygdala Signaling during Foraging in a Hazardous Environment
Abstract
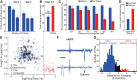
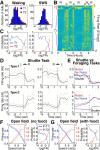
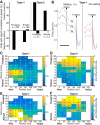
We recorded basolateral amygdala (BL) neurons in a seminaturalistic foraging task. Rats had to leave their nest to retrieve food in an elongated arena inhabited by a mechanical predator. There were marked trial-to-trial variations in behavior. After poking their head into the foraging arena and waiting there for a while, rats either retreated to their nest or initiated foraging. Before initiating foraging, rats waited longer on trials that followed failed than successful trials indicating that prior experience influenced behavior. Upon foraging initiation, most principal cells (Type-1) reduced their firing rate, while in a minority (Type-2) it increased. When rats aborted foraging, Type-1 cells increased their firing rates, whereas in Type-2 cells it did not change. Surprisingly, the opposite activity profiles of Type-1 and Type-2 units were also seen in control tasks devoid of explicit threats or rewards. The common correlate of BL activity across these tasks was movement velocity, although an influence of position was also observed. Thus depending on whether rats initiated movement or not, the activity of BL neurons decreased or increased, regardless of whether threat or rewards were present. Therefore, BL activity not only encodes threats or rewards, but is closely related to behavioral output. We propose that higher order cortical areas determine task-related changes in BL activity as a function of reward/threat expectations and internal states. Because Type-1 and Type-2 cells likely form differential connections with the central amygdala (controlling freezing), this process would determine whether movement aimed at attaining food or exploration is suppressed or facilitated. Significance statement: For decades, amygdala research has been dominated by pavlovian and operant conditioning paradigms. This work has led to the view that amygdala neurons signal threats or rewards, in turn causing defensive or approach behaviors. However, the artificial circumstances of conditioning studies bear little resemblance to normal life. In natural conditions, subjects are simultaneously presented with potential threats and rewards, forcing them to engage in a form of risk assessment. We examined this process using a seminaturalistic foraging task. In constant conditions of threats and rewards, amygdala activity could be high or low, depending on the rats' decisions on a given trial. Therefore, amygdala activity does not only encode threats or rewards but is also closely related to behavioral output.
Keywords: amygdala; approach; defensive behaviors; reward; threat.
Copyright © 2015 the authors 0270-6474/15/3512994-12$15.00/0.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
