Intermediate Progenitors Facilitate Intracortical Progression of Thalamocortical Axons and Interneurons through CXCL12 Chemokine Signaling
- PMID: 26400936
- PMCID: PMC6605439
- DOI: 10.1523/JNEUROSCI.1488-15.2015
Intermediate Progenitors Facilitate Intracortical Progression of Thalamocortical Axons and Interneurons through CXCL12 Chemokine Signaling
Abstract
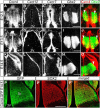
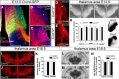
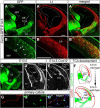
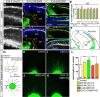
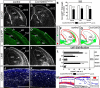
Glutamatergic principal neurons, GABAergic interneurons and thalamocortical axons (TCAs) are essential elements of the cerebrocortical network. Principal neurons originate locally from radial glia and intermediate progenitors (IPCs), whereas interneurons and TCAs are of extrinsic origin. Little is known how the assembly of these elements is coordinated. C-X-C motif chemokine 12 (CXCL12), which is known to guide axons outside the neural tube and interneurons in the cortex, is expressed in the meninges and IPCs. Using mouse genetics, we dissected the influence of IPC-derived CXCL12 on TCAs and interneurons by showing that Cxcl12 ablation in IPCs, leaving meningeal Cxcl12 intact, attenuates intracortical TCA growth and disrupts tangential interneuron migration in the subventricular zone. In accordance with strong CXCR4 expression in the forming thalamus and TCAs, we identified a CXCR4-dependent growth-promoting effect of CXCL12 on TCAs in thalamus explants. Together, our findings indicate a cell-autonomous role of CXCR4 in promoting TCA growth. We propose that CXCL12 signals from IPCs link cortical neurogenesis to the progression of TCAs and interneurons spatially and temporally. Significance statement: The cerebral cortex exerts higher brain functions including perceptual and emotional processing. Evolutionary expansion of the mammalian cortex is mediated by intermediate progenitors, transient amplifying cells generating cortical excitatory neurons. During the peak period of cortical neurogenesis, migrating precursors of inhibitory interneurons originating in subcortical areas and thalamic axons invade the cortex. Although defects in the assembly of cortical network elements cause neurological and mental disorders, little is known how neurogenesis, interneuron recruitment, and axonal ingrowth are coordinated. We demonstrate that intermediate progenitors release the chemotactic cytokine CXCL12 to promote intracortical interneuron migration and growth of thalamic axons via the cognate receptor CXCR4. This paracrine signal may ensure thalamocortical connectivity and dispersion of inhibitory neurons in the rapidly growing cortex.
Keywords: CXCL12/CXCR4; cortical development; intermediate/basal progenitors; interneuron; thalamocortical axons; thalamus.
Copyright © 2015 the authors 0270-6474/15/3513053-11$15.00/0.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
