Flexible Use of Predictive Cues beyond the Orbitofrontal Cortex: Role of the Submedius Thalamic Nucleus
- PMID: 26400947
- PMCID: PMC6605447
- DOI: 10.1523/JNEUROSCI.1237-15.2015
Flexible Use of Predictive Cues beyond the Orbitofrontal Cortex: Role of the Submedius Thalamic Nucleus
Abstract
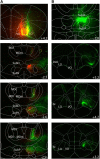
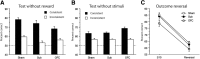
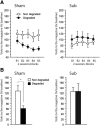
The orbitofrontal cortex (OFC) is known to play a crucial role in learning the consequences of specific events. However, the contribution of OFC thalamic inputs to these processes is largely unknown. Using a tract-tracing approach, we first demonstrated that the submedius nucleus (Sub) shares extensive reciprocal connections with the OFC. We then compared the effects of excitotoxic lesions of the Sub or the OFC on the ability of rats to use outcome identity to direct responding. We found that neither OFC nor Sub lesions interfered with the basic differential outcomes effect. However, more specific tests revealed that OFC rats, but not Sub rats, were disproportionally relying on the outcome, rather than on the discriminative stimulus, to guide behavior, which is consistent with the view that the OFC integrates information about predictive cues. In subsequent experiments using a Pavlovian contingency degradation procedure, we found that both OFC and Sub lesions produced a severe deficit in the ability to update Pavlovian associations. Altogether, the submedius therefore appears as a functionally relevant thalamic component in a circuit dedicated to the integration of predictive cues to guide behavior, previously conceived as essentially dependent on orbitofrontal functions. Significance statement: In the present study, we identify a largely unknown thalamic region, the submedius nucleus, as a new functionally relevant component in a circuit supporting the flexible use of predictive cues. Such abilities were previously conceived as largely dependent on the orbitofrontal cortex. Interestingly, this echoes recent findings in the field showing, in research involving an instrumental setup, an additional involvement of another thalamic nuclei, the parafascicular nucleus, when correct responding requires an element of flexibility (Bradfield et al., 2013a). Therefore, the present contribution supports the emerging view that limbic thalamic nuclei may contribute critically to adaptive responding when an element of flexibility is required after the establishment of initial learning.
Keywords: Pavlovian associations; adaptive behaviors; degradation; differential outcome effect; rat.
Copyright © 2015 the authors 0270-6474/15/3513183-11$15.00/0.
Figures




References
-
- Alcaraz F, Naneix F, Desfosses E, Marchand AR, Wolff M, Coutureau E. Dissociable effects of anterior and mediodorsal thalamic lesions on spatial goal-directed behavior. Brain Struct Funct. 2014 Advance online publication. Retrieved Aug. 25, 2015. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
