Aging-Related Hyperexcitability in CA3 Pyramidal Neurons Is Mediated by Enhanced A-Type K+ Channel Function and Expression
- PMID: 26400949
- PMCID: PMC4579378
- DOI: 10.1523/JNEUROSCI.0193-15.2015
Aging-Related Hyperexcitability in CA3 Pyramidal Neurons Is Mediated by Enhanced A-Type K+ Channel Function and Expression
Abstract
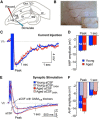
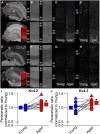
Aging-related impairments in hippocampus-dependent cognition have been attributed to maladaptive changes in the functional properties of pyramidal neurons within the hippocampal subregions. Much evidence has come from work on CA1 pyramidal neurons, with CA3 pyramidal neurons receiving comparatively less attention despite its age-related hyperactivation being postulated to interfere with spatial processing in the hippocampal circuit. Here, we use whole-cell current-clamp to demonstrate that aged rat (29-32 months) CA3 pyramidal neurons fire significantly more action potentials (APs) during theta-burst frequency stimulation and that this is associated with faster AP repolarization (i.e., narrower AP half-widths and enlarged fast afterhyperpolarization). Using a combination of patch-clamp physiology, pharmacology, Western blot analyses, immunohistochemistry, and array tomography, we demonstrate that these faster AP kinetics are mediated by enhanced function and expression of Kv4.2/Kv4.3 A-type K(+) channels, particularly within the perisomatic compartment, of CA3 pyramidal neurons. Thus, our study indicates that inhibition of these A-type K(+) channels can restore the intrinsic excitability properties of aged CA3 pyramidal neurons to a young-like state. Significance statement: Age-related learning deficits have been attributed, in part, to altered hippocampal pyramidal neuronal function with normal aging. Much evidence has come from work on CA1 neurons, with CA3 neurons receiving comparatively less attention despite its age-related hyperactivation being postulated to interfere with spatial processing. Hence, we conducted a series of experiments to identify the cellular mechanisms that underlie the hyperexcitability reported in the CA3 region. Contrary to CA1 neurons, we demonstrate that postburst afterhyperpolarization is not altered with aging and that aged CA3 pyramidal neurons are able to fire significantly more action potentials and that this is associated with faster action potential repolarization through enhanced expression of Kv4.2/Kv4.3 A-type K(+) channels, particularly within the cell bodies of CA3 pyramidal neurons.
Keywords: A-type K+ channels; CA3; Kv4.2/Kv4.3; action potential repolarization; aging; pyramidal neurons.
Copyright © 2015 the authors 0270-6474/15/3513206-13$15.00/0.
Figures






Similar articles
-
Calcium-activated afterhyperpolarizations regulate synchronization and timing of epileptiform bursts in hippocampal CA3 pyramidal neurons.J Neurophysiol. 2006 Dec;96(6):3028-41. doi: 10.1152/jn.00434.2006. Epub 2006 Sep 13. J Neurophysiol. 2006. PMID: 16971683
-
Intracellular activities related to in vitro hippocampal sharp waves are altered in CA3 pyramidal neurons of aged mice.Neuroscience. 2014 Sep 26;277:474-85. doi: 10.1016/j.neuroscience.2014.07.048. Epub 2014 Aug 1. Neuroscience. 2014. PMID: 25088916
-
Cell-type specific depression of neuronal excitability in rat hippocampus by activation of ATP-sensitive potassium channels.Eur Biophys J. 2002 Oct;31(6):467-77. doi: 10.1007/s00249-002-0241-3. Epub 2002 Aug 9. Eur Biophys J. 2002. PMID: 12355256
-
Intrinsic Hippocampal Excitability Changes of Opposite Signs and Different Origins in CA1 and CA3 Pyramidal Neurons Underlie Aging-Related Cognitive Deficits.Front Syst Neurosci. 2016 Jun 9;10:52. doi: 10.3389/fnsys.2016.00052. eCollection 2016. Front Syst Neurosci. 2016. PMID: 27375440 Free PMC article. Review.
-
Pharmacological and molecular enhancement of learning in aging and Alzheimer's disease.J Physiol Paris. 2006 Mar-May;99(2-3):180-92. doi: 10.1016/j.jphysparis.2005.12.079. Epub 2006 Feb 3. J Physiol Paris. 2006. PMID: 16458491 Review.
Cited by
-
Impaired pattern separation in Tg2576 mice is associated with hyperexcitable dentate gyrus caused by Kv4.1 downregulation.Mol Brain. 2021 Mar 30;14(1):62. doi: 10.1186/s13041-021-00774-x. Mol Brain. 2021. PMID: 33785038 Free PMC article.
-
Delayed formation of neural representations of space in aged mice.Aging Cell. 2023 Sep;22(9):e13924. doi: 10.1111/acel.13924. Epub 2023 Jul 25. Aging Cell. 2023. PMID: 37491802 Free PMC article.
-
Long Non-Coding RNAs in Neuronal Aging.Noncoding RNA. 2018 Apr 18;4(2):12. doi: 10.3390/ncrna4020012. Noncoding RNA. 2018. PMID: 29670042 Free PMC article. Review.
-
Neurons and glial cells acquire a senescent signature after repeated mild traumatic brain injury in a sex-dependent manner.Front Neurosci. 2022 Nov 3;16:1027116. doi: 10.3389/fnins.2022.1027116. eCollection 2022. Front Neurosci. 2022. PMID: 36408415 Free PMC article.
-
Regulation of intrinsic excitability: Roles for learning and memory, aging and Alzheimer's disease, and genetic diversity.Neurobiol Learn Mem. 2019 Oct;164:107069. doi: 10.1016/j.nlm.2019.107069. Epub 2019 Aug 20. Neurobiol Learn Mem. 2019. PMID: 31442579 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
